Rimegepant: Pharmacodynamic Properties, Pharmacokinetic Properties and Drug Interactions
May 9,2025
Background
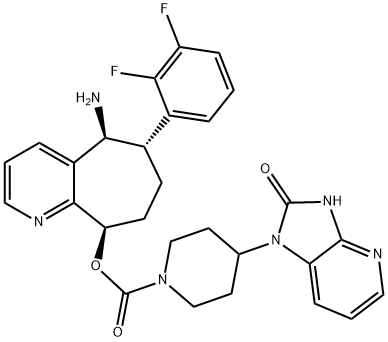
Rimegepant (Figure 1) is a small molecule inhibitor of the receptor for the calcitonin gene-related peptide(CGRP), which is believed to play a role in the pathogenesis of migraine headaches. CGRP is a potent vasodilator and pain-signaling neurotransmitter that is found throughout the central and peripheral nervous system but is particularly common in trigeminal ganglia. Levels of CGRP are elevated during episodes of migraine headache, and administration of the peptide can induce migraines in susceptible patients. For this reason, approaches to inhibition of CGRP signaling were developed as potential therapies for migraine, both as preventive therapies to decrease the rate of migraine as well as for treatment of acute attacks. Several monoclonal antibodies that block CGRP or its receptor are approved for use in prevention of migraines and two small molecule inhibitors of the CGRP receptor (the “gepants”: ubrogepant and rimegepant) are available for treatment of acute migraine. In several randomized, placebo controlled trials, rimegepant in doses of 75 mg was found to increase the rate of being free of headache pain by two hours after [20% to 21% vs 11% to 12% with placebo] and free of the most bothersome other symptoms [35% to 38% vs 25% to 27% with placebo].Rimegepant was approved for treatment of acute migraine in the United States in 2020, the second oral CGRP receptor antagonist approved for this indication. Rimegepant is available in orally disintegrating tablets of 75 mg under the brand name Nurtec-ODT. The recommended dose is 75 mg orally as soon as possible after onset of migraine. The dose should not exceed 75 mg during any 24 hour period. Rimegepant can be used by patients receiving preventive therapy with monoclonal antibodies to CGRP or its receptor. Rimegepant is generally well tolerated with side effects of nausea, dizziness, somnolence and dry mouth that are generally uncommon (<5%),transient and mild to moderate in severity. Hypersensitivity reactions (largely rash and dyspnea) have arisen in rare cases, but more severe adverse events have not been reported.[1]

Pharmacodynamic Properties of Rimegepant
In vitro, rimegepant effectively antagonized CGRP activity at both the CGRP receptor and the structurally related amylin 1(AMY1) receptor, but was ≈30-fold more effective at blocking the CGRP receptor. Rimegepant inhibited CGRP induced currents with inhibition potencies (pIC50 values) of 11.30 for the CGRP receptor and 9.91 for the AMY1 recepto. In non-clinical assays, rimegepant antagonized CGRP mediated increases in facial blood fow (a surrogate marker for intracranial artery dilation), with 75% inhibition at ≈700nmol/L. The inhibitory constant (Ki) of rimegepant against the CGRP receptor was 0.027 nmol/L (6.9% fraction unbound).
Rimegepant, at concentrations up to 10 μmol/L, exhibited no active vasoconstrictive properties in ex vivo human coronary or cerebral arteries. Conversely, sumatriptan exhibited progressive, concentration-dependent constriction of arteries, a known limitation of the triptan class. The absence of rimegepant-induced vasoconstriction was not due to arteriolar defect, as each vessel exhibited vasoconstriction in the presence of 10 μmol/L serotonin. In primates, exposure to high concentrations of rimegepant (i.e.10xgreater than those observed in humans at the recommended dose of 75 mg) had no effect on haemodynamicor electrocardiographic parameters. There were also no changes in cardiovascular parameters after 9 months of daily administration of rimegepant 50 mg/kg (i.e.≈20×the human therapeutic dose).
In healthy volunteers, a single therapeutic (75 mg) or supratherapeutic (300 mg) dose of rimegepant did not prolong the QT interval to any clinically relevant extent. There were no clinically relevant differences in resting blood pressure when rimegepant was coadministered with sumatriptan (two 6 mg tablets taken 1 h apart) relative to sumatriptan alone.[2]
Pharmacokinetic Properties of Rimegepant[2]
The ODT formulation of rimegepant administered sublingually was bioequivalent to the conventional oral tablet formulation of rimegepant in a randomized, open-label, phase I trial in healthy volunteers (n = 35). The 90% CIs of geometric mean ratios for area under the concentration-time curve (AUC) over the dosing interval (AUC0–τ), AUC from zero to infinity (AUC0–∞) and maximum plasma concentration (Cmax) were within the predefined range for bioequivalence (80–125%). The least squares mean time to Cmax (tmax) was 1.48 h with the ODT formulation versus 1.92 h with the conventional tablet formulation (p=0.0021), a diference of 26 min. Rimegepant exhibits greater than dose-proportional linear pharmacokinetics following a single oral dose. The absolute oral bioavailability of rimegepant is ≈64%. When rimegepant was administered with a high-fat meal, tmax was prolonged by ≈1–1.5 h, Cmax was reduced by 42–53% and AUC was reduced by 32–38%. The clinical impact of reduced rimegepant exposure due to food is unknown. However, in clinical trials, rimegepant was administered without regard to food. Rimegepant has a steady-state volume of distribution of 120 L and is highly (≈96%) bound to plasma proteins. Rimegepant is metabolized primarily by CYP3A4 and, to a lesser extent, by CYP2C9, with no major metabolites detected in plasma. It is eliminated mostly (≈77% of the dose) as unchanged drug, with 78% of the dose recovered in the faeces (42% as unchanged drug) and 24% in the urine (51% as unchanged drug). Rimegepant has an elimination half-life of ≈11h.[3,4]
The pharmacokinetics of rimegepant were not affected by sex, age (i.e. non-elderly 18–45 years vs elderly ≥65 years), race/ethnicity (including Chinese vs non-Chinese ethnicity), body weight, migraine status, CYP2C9 genotype, impaired kidney function (mild, moderate or severe), or mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. Rimegepant has not been studied in patients with end-stage renal disease or patients on dialysis; its use in patients with ESRD should be avoided; theuse of rimegepant should be avoided in patients with severehepatic impairment.[3,4]
Drug Interactions
Rimegepant is a substrate of CYP3A4, CYP2C9, P-gp and BCRP, and a weak inhibitor of CYP3A4. Coadministration of rimegepant with strong CYP3A4 inhibitors and strong or moderate CYP3A4 inducers is not recommended.Another dose of rimegepant within 48 h should be avoided when the drug is coadministered with moderate CYP3A4inhibitors or strong P-gp inhibitors. Rimegepant is an inhibitor of OATP1B3, OCT2 and MATE1, and a weak inhibitor of OATP1B1 and OAT3. No clinical drug interactions are expected for rimegepant with OAT1PB3 or OCT2 at clinically relevant concentrations. There were no significant pharmacokinetic interactions when rimegepant was coadministered with oral contraceptives (norelgestromin, ethinyl estradiol), midazolam (a sensitive CYP3A4 substrate), metformin (a MATE1 substrate) or sumatriptan.[2]
References
[1] Rimegepant. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; January 12, 2025.
[2]Blair HA. Rimegepant: A Review in the Acute Treatment and Preventive Treatment of Migraine [published correction appears in CNS Drugs. 2023 Jul;37(7):661. doi: 10.1007/s40263-023-01019-2.]. CNS Drugs. 2023;37(3):255-265. doi:10.1007/s40263-023-00988-8
[3]Biohaven Pharmaceuticals. NURTEC ODT (rimegepant) orallydisintegrating tablets, for sublingual or oral use: US prescribing information. 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9ef08e09-1098-35cc-e053-2a95a90a3e1d.Accessed 23 Jan 2023.
[4]European Medicines Agency. VYDURA 75 mg oral lyophilisate:EU summary of product characteristics. 2022.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical Engineering1,3-Dimethoxybenzene, produced from phenol and methanol, serves as an initiator in polymer/resin synthesis, a solvent, and an extractant.....
May 9,2025Chemical MaterialsRimegepant
1289023-67-1You may like
Rimegepant manufacturers
- Rimegepant
-

- $5.00/ KG
- 2025-12-12
- CAS:1289023-67-1
- Min. Order: 1KG
- Purity: 99% hplc
- Supply Ability: 500TONS
- Rimegepant
-
- $0.00 / 10g
- 2025-09-22
- CAS:1289023-67-1
- Min. Order: 10g
- Purity: 99%
- Supply Ability: 5kgs
- Rimegepant
-

- $20.00 / 1KG
- 2025-06-20
- CAS:1289023-67-1
- Min. Order: 1KG
- Purity: 0.99
- Supply Ability: 20