| Identification | Back Directory | [Name]
Chlorprothixene | [CAS]
113-59-7 | [Synonyms]
CPX
CPO
CPOX
n714
n714c
Paxyl
mk184
N 714
N 714C
MK 184
Truxal
Truxil
TARDAN
Cloxan
Trictal
Tarasan
ro4-0403
Tactaran
Taractan
Rentovet
Vetacalm
Iaractan
Traquilan
Ro 4-0403
n#714trans
Ro-4-04033
Chlothixen
Truxaletten
Chlorprotixen
Chlorprotixene
Chlorprotixine
Chlorprothixen
Chlorprothixine
CHLORPROTHIXENE
Chlorthiaxanthen
Chloroprothixene
Carboxypeptidase O
(Z)-chlorprothixene
Chlorprothixene HCI
cis-Chlorprothixene
alpha-Chlorprothixene
9H-thioxanthene,1-propanaminederiv.
9H-Thioxanthene, 1-propanamine deriv.
ANTI-CPO (N-TERM) antibody produced in rabbit
cis-2-Chloro-9-(3-dimethylaminopropylidene)thioxanthene
2-chloro-9-(omega-di-methylaminopropylidene)thioxanthene
2-chloro-n,n-dimethyl-3-thioxanthen-9-ylidenepropylamine
2-Chloro-9-(omega-(dimethylamino)propylidene)thioxanthene
thioxanthene-Δ9,γ-propylamine,2-chloro-N,N-dimethyl-,(Z)-
trans-2-chloro-9-(3-dimethylaminopropylidene)thiaxanthene
(z)-2-chloro-9-(omega-dimethylaminopropylidene)thioxanthene
alpha-2-chloro-10-(3-dimethylaminopropylidene)-thiaxanthene
2-chloro-3-(dibenzo(b,e)thiin-10-ylidene)propyldimethylamine
3-(2-chlorothioxanthen-9-ylidene)-n,n-dimethyl-propan-1-amine
(alpha-2-chloro-9-omega-dimethylamino-propylamine)thioxanthene
(alpha-2-Chloro-9-omega-dimethylamino-propylidene)thioxanthene
2-chloro-n,n-dimethyl-gamma)-propylamin(z)-thioxanthene-delta(
2-chloro-n,n-dimethylthioxanthene-delta(sup9),gamma-propylamine
gamma-propylamine,2-chloro-n,n-dimethyl-thioxanthene-delta(sup9
3-(2-chloro-9h-thioxanthen-9-ylidene)-n,n-dimethyl-1-propanamine
3-(2-chloro-9H-thioxanthen-9-ylidene)-N-,N-dimethyl-1-propanamine
2-chloro-n,n-dimethyl-gamma-propylamin(z)-thioxanthene-delta(sup9
n-dimethyl-3-(2-chloro-9h-thioxanthen-9-ylidene)-(z)-1-propanamin
Thioxanthene-delta9,gamma-propylamine, 2-chloro-N,N-dimethyl-, (Z)-
(Z)-3-(2-chloro-9H-thioxanthen-9-ylidene)-N,N-dimethyl-1-propanamine
(3E)-3-(2-Chloro-9H-thioxanthen-9-ylidene)-N,N-dimethyl-1-propanamine
(3Z)-3-(2-chloro-9H-thioxanthen-9-ylidene)-N,N-dimethyl-1-propanamine
2-Chloro-N,N-dimethylthioxanthene-delta9, gamma-propylamine
1-Propanamine, 3-(2-chloro-9H-thioxanthen-9-ylidene)-N,N-dimethyl-, (Z)-
(Z)-2-Chloro-N,N-dimethylthioxanthene-delta(sup9),(supgamma )-propylamine
1-Propanamine, 3-(2-chloro-9H-thioxanthen-9-ylidene)-N,N-dimethyl-, (3Z)-
Thioxanthene-delta9, gamma-propylamine, 2-chloro-N,N-dimethyl-
(Z)-2-Chloro-N,N-dimethylthioxanthene-(GR D)9,gamma-propylamine | [EINECS(EC#)]
204-032-8 | [Molecular Formula]
C18H18ClNS | [MDL Number]
MFCD00869180 | [MOL File]
113-59-7.mol | [Molecular Weight]
315.86 |
| Chemical Properties | Back Directory | [Melting point ]
97-98° | [Boiling point ]
160 °C(Press: 0.04 Torr) | [density ]
1.1048 (rough estimate) | [refractive index ]
1.6000 (estimate) | [storage temp. ]
-20°C | [solubility ]
DMSO : 33.33 mg/mL (105.52 mM; Need ultrasonic)H2O : < 0.1 mg/mL (insoluble) | [form ]
powder to crystal | [pka]
pKa 8.4(H2O) (Uncertain) | [color ]
White to Orange to Green | [Water Solubility ]
385.8ug/L(22.5 ºC) | [Stability:]
Hygroscopic | [InChI]
1S/C18H18ClNS/c1-20(2)11-5-7-14-15-6-3-4-8-17(15)21-18-10-9-13(19)12-16(14)18/h3-4,6-10,12H,5,11H2,1-2H3/b14-7- | [InChIKey]
WSPOMRSOLSGNFJ-AUWJEWJLSA-N | [SMILES]
S1c2c(cc(cc2)Cl)\C(=C/CCN(C)C)\c3c1cccc3 | [NIST Chemistry Reference]
Chlorprothixene(113-59-7) |
| Hazard Information | Back Directory | [Uses]
muscle relaxant (skeletal) | [Description]
In 2002, the American Association of Poison Control Centers’
Toxic Exposure Surveillance System reported 5224 human
exposures to phenothiazines, thioxanthenes, and other neuroleptic
medications; 3691 were in adults and 808 in children.
Unintentional and intentional exposures accounted for 43.7
and 47.8%, respectively. There were 417 (8.0%) adverse drug
reactions reported. Thioxanthenes are chemical compounds in
which the oxygen atom in xanthene is replaced with a sulfur
atom. They are also related to phenothiazines. Several derivatives
are used as typical antipsychotics in the treatment of
schizophrenia and other psychoses. The thioxanthenes, as
a class, are closely related chemically to the phenothiazines. The
major structural difference is that the nitrogen at position 10 in
the phenothiazines is replaced by a carbon atom with a double
bond to the side chain, as shown in the chemical structure of
flupenthixol, which has a double-bonded carbon in the number
10 position. Clopenthixol is a typical antipsychotic drug of the
thioxanthenes class and a racemic mixture of cis and trans
isomers. Zuclopenthixol, the pure cis isomer, has been much
more widely used. Both drugs are equally effective as antipsychotics
and have similar adverse effect profiles, but clopenthixol
is half as active on a milligram-to-milligram basis and appears
to produce more sedation in comparison. | [Chemical Properties]
Pale yellow crystalline powder. Melting point 97-98°C. Insoluble in ethanol, ether, and chloroform. Its hydrochloride ([6469-93-8]), melting point 221°C, is soluble in water. | [Originator]
aractan, Roche, France ,1960 | [Definition]
ChEBI: (Z)-chlorprothixene is a chlorprothixene in which the double bond adopts a (Z)-configuration. It is an enantiomer of an (E)-chlorprothixene. | [Manufacturing Process]
Chlorprothixene may be prepared as described in US Patent 2,951,082.
Magnesium turnings, 4.86 g (0.2 g-atom) was placed in a 500 ml reaction
flask fitted with a mercury sealed stirrer, reflux condenser and a dropping
funnel. Tetrahydrofuran, 50 ml and calcium hydride, 500 mg, were added.
Ethyl bromide, 2.18 g and a crystal of iodine then were added. A vigorous
reaction set in that evolved sufficient heat to induce refluxing. After 5
minutes, a solution of 3-dimethylaminopropyl chloride (dried over calcium
hydride) in 50 ml of tetrahydrofuran was added to the refluxing solution at
such a rate that gentle refluxing was maintained. The addition required 25
minutes.
The reaction mixture was stirred at reflux for an additional 30 minutes when
nearly all of the magnesium had dissolved and determination of magnesium in
an aliquot of the solution showed that an 82% yield of Grignard reagent had
been obtained. The reaction mixture was cooled in an ice bath and stirred
while 24.67 g (0.1 mol) of 2-chlorothiaxanthone was added over a period of
10 minutes. The reaction was stirred at room temperature for 30 minutes
then allowed to stand overnight in the refrigerator. The tetrahydrofuran was
evaporated at 50°C under reduced pressure. Benzene, 150 ml, was added to
the residue.
The mixture was hydrolyzed in the cold by the dropwise addition of 50 ml of
water. The benzene layer was separated by decantation and the gelatinous
precipitate washed with two 100 ml portions of benzene.
The precipitate was then mixed with diatomaceous earth, collected on a filter,
and washed with water and extracted with two 100 ml portions of boiling
benzene. The aqueous filtrate was extracted with 50 ml of benzene, the
combined benzene extracts washed with water and evaporated to dryness
under reduced pressure. The crystalline residue, MP 140° to 147°C, weighed
30.8 g. Recrystallization from a mixture of benzene and hexane gave 27.6 g
(83%) of 2-chloro-10-(3-dimethylaminopropyl)-10-hydroxythiaxanthene, MP
152° to 154°C. Analytically pure material from another experiment melted at
153° to 154°C.
2-Chloro-10-(3-dimethylaminopropyl)-10-hydroxythiaxanthene, 3.34 g (0.01
mol) obtained as described was dissolved in 15 ml of dry, alcohol-free
chloroform. Acetyl chloride, 2.36 g (0.03 mol) was added and the clear yellow
solution was refluxed for one hour in a system protected by a drying tube.
The solvent then was evaporated on the steam bath under reduced pressure
and the residue dissolved in absolute alcohol. The hydrochloride of 2-chloro-
10-(3-dimethylaminopropylidene)-thiaxanthene was precipitated by the
cautious addition of absolute ether. After drying at 70°C the yield of white
crystalline 2-chloro10-(3-dimethylaminopropylidene)-thiaxanthene
hydrochloride, MP 189 to 190°C (to a cloudy melt), was 3.20 g (90%). This
material is a mixture of geometric isomers.
Trans-2-chloro-9-(ω-dimethylamino-propylidene)-thioxanthene [MP 98°C, MP
of the hydrochloride 225°C (corr.)], is a valuable medicinal agent, being used
as a tranquilizer and antiemetic agent, whereas the corresponding cis isomer
(MP 44°C, MP of the hydrochloride 209°C) is not useful for these indications,
as described in US Patent 3,115,502, which describes procedures for
conversion of the cis to the trans form. | [Brand name]
Taractan
(Roche). | [Therapeutic Function]
Tranquilizer | [Synthesis]
Chlorprothixene, 2-chloro-9[(1-dimethylamino)-3-propyliden]thioxan�thene (6.2.7), has been proposed to synthesize starting from 2-chlorothixantone (6.2.3). The
initial 2-chlorothixantone (6.2.3) is prepared from 2-mercaptobenzoic acid, the reaction of which with 1-bromo-4-chlorobenzene forms 2-(4-chlorophenylthio)benzoic acid (6.2.1),
which upon reaction with phosphorous pentachloride transforms into acid chloride (6.2.2),
and further undergoes intramolecular cyclization with the use of aluminum chloride to give 2-
chlorthioxantone (6.2.3) [32]. An alternative way of making 2-chlorthioxantone (6.2.3) is by
making 2-(4-chlorophenylthio)benzoic acid (6.2.1) by reacting 2-iodobenzoic acid with 4-
chlorothiophenol [33]. The resulting 2-chlorthioxantone (6.2.3) is reacted as a carbonyl com�ponent with either 3-dimethylaminopropylmagnesiumbromide [33], or with
allylmagnesiumbromide [34¨C36], giving the corresponding tertiary alcohol (6.2.4) or (6.2.5).
Dehydration of the first is accomplished by acylation of the tertiary hydroxyl group using
acetyl chloride and the subsequent pyrolysis of the formed acetate, which leads to the desired
chlorprothixene (6.2.7).
Dehydration of the tertiary alcohol (6.2.5) is accomplished by chlorination of the terti�ary alcohol group by thionyl chloride, forming the diene 2-chloro-9-(3-propen-1-
iliden)thioxanthene (6.2.6), the addition to which of dimethylamine at high temperature
forms the desired chlorprothixene (6.2.7).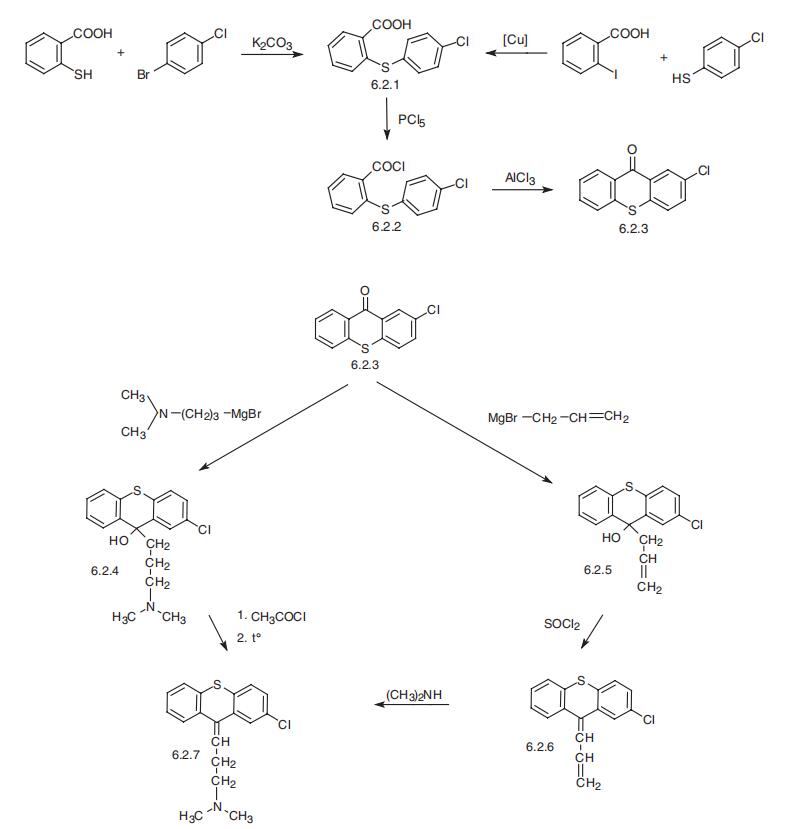
| [Environmental Fate]
Long-range transport: handling of thioxanthenes should
only be performed by personnel trained and familiar
with handling potent active pharmaceutical ingredients.
In case of handling, avoid inhalation and contact with
skin, eyes, and clothing, as these materials may be an
irritant. These substances are considered nonhazardous
for transport. | [Toxicity evaluation]
Thioxanthenes work primarily by blocking postsynaptic
dopamine-mediated neurotransmission by binding to dopamine
(DA-1 and DA-2) receptors. In addition to significant
antidopaminergic action, the thioxanthenes also possess weak
anticholinergic and serotonergic blockade, moderate a-adrenergic
blockade, quinidine-like effects, and depress the release of
most hypothalamic and hypophyseal hormones. Thioxanthenes
may also inhibit presynaptic dopamine autoreceptors.
The concentration of prolactin is increased due to
blockade of prolactin inhibitory factor, which inhibits the
release of prolactin from the pituitary gland. Chlorprothixene
also inhibits the medullary chemoreceptor trigger zone to
produce an antiemetic effect; and is thought to cause an indirect
reduction of stimuli to the brain stem reticular system to
produce a sedative effect. |
| Safety Data | Back Directory | [WGK Germany ]
WGK 1 | [HS Code ]
2934.99.3000 | [Storage Class]
6.1C - Combustible acute toxic Cat.3
toxic compounds or compounds which causing chronic effects | [Hazard Classifications]
Acute Tox. 3 Oral
STOT SE 3 | [Hazardous Substances Data]
113-59-7(Hazardous Substances Data) | [Toxicity]
LD50 oral in rabbit: 182mg/kg |
|
|