| Identification | More | [Name]
TOLAZAMIDE | [CAS]
1156-19-0 | [Synonyms]
1-[HEXAHYDRO-1H-AZEPIN-1-YL]-3-[P-TOLUENESULFONYL]UREA
LABOTEST-BB LT00772329
N-[[(HEXAHYDRO-1H-AZEPINYL)AMINO]CARBONYL]-4-METHYLBENZENESULFONAMIDE
SALOR-INT L254916-1EA
TOLAZAMIDE
U-17835
1-(hexahydro-1-azepinyl)-3-p-tolylsulfonylurea
1-(hexahydro-1h-azepin-1-yl)-3-(p-tolylsulfonyl)-ure
1-(hexahydro-1h-azepin-1-yl)-3-(p-tolylsulfonyl)urea
4-(p-tolylsulfonyl)-1,1-hexamethylenesemicarbazide
diabewas
n-(((hexahydro-1h-azepin-1-yl)-amino)carbonyl)-4-methyl-benzenesulfonamid
n-(p-toluenesulfonyl)-n’-hexamethyleniminourea
n,((hexahydro-1h-azepin-1-yl)amino)carbonyl)-4-methyl-benzenesulfonamid
n-[[(hexahydro-1h-azepin-1-yl)-amino]carbonyl]-4-methylbenzenesulfonamide
nci-c03327
norglycin
nsc-70762
tolanase
tolazolamide | [EINECS(EC#)]
214-588-3 | [Molecular Formula]
C14H21N3O3S | [MDL Number]
MFCD00083504 | [Molecular Weight]
311.4 | [MOL File]
1156-19-0.mol |
| Chemical Properties | Back Directory | [Melting point ]
162-164°C | [Boiling point ]
300°C (rough estimate) | [density ]
1.2228 (rough estimate) | [refractive index ]
1.6740 (estimate) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Very slightly soluble in water; freely soluble in chloroform; soluble in acetone; slightly soluble in ethanol (96%). | [form ]
Solid | [pka]
3.6(at 25℃) | [color ]
White to Off-White | [Water Solubility ]
65.4mg/L(30 ºC) | [Major Application]
pharmaceutical (small molecule) | [InChI]
InChI=1S/C14H21N3O3S/c1-12-6-8-13(9-7-12)21(19,20)16-14(18)15-17-10-4-2-3-5-11-17/h6-9H,2-5,10-11H2,1H3,(H2,15,16,18) | [InChIKey]
OUDSBRTVNLOZBN-UHFFFAOYSA-N | [SMILES]
C1(S(NC(NN2CCCCCC2)=O)(=O)=O)=CC=C(C)C=C1 | [CAS DataBase Reference]
1156-19-0(CAS DataBase Reference) | [EPA Substance Registry System]
Tolazamide (1156-19-0) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed. | [Safety Statements ]
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [RTECS ]
YT4400000
| [HS Code ]
2935904000 | [Storage Class]
11 - Combustible Solids | [Hazardous Substances Data]
1156-19-0(Hazardous Substances Data) | [Toxicity]
LD50 in rats, mice (mg/kg): >5000 orally, 2239 i.p. (Dulin) |
| Hazard Information | Back Directory | [General Description]
White to off-white crystalline powder. Odorless or with a slight odor. | [Reactivity Profile]
TOLAZAMIDE(1156-19-0) is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). This chemical is incompatible with acids. . | [Air & Water Reactions]
This chemical may be sensitive to prolonged exposure to air. Insoluble in water. | [Fire Hazard]
Flash point data for this chemical are not available; however, TOLAZAMIDE is probably combustible. | [Description]
Tolazamide is a first generation sulfonylurea that inhibits sulfonylurea receptor 1 (SUR1) linked to the inwardly rectifying potassium channel (KIR6.2; IC50 = 4.2 μM in HEK293 cells transfected with the human receptor).1 It has no effect on glucose uptake in L6 rat skeletal muscle cells when used at a concentration of 0.6 mg/mL but enhances glucose uptake two-fold when used in combination with insulin.2 In vivo, tolazamide (128 mg/kg) reduces glomerulosclerosis and albumin excretion in a rat model of insulin-dependent diabetes induced by streptozotocin (Item No. 13104).3 Formulations containing tolazamide have been used in the treatment of type 2 diabetes. | [Chemical Properties]
White Solid | [Originator]
Tolinase,Upjohn,Italy,1964 | [Uses]
Labelled Tolazamide, an antidiabetic. | [Uses]
This drug is also a derivative of first generation of sulfonylurea, and it possesses stimulatory
action on β-cells in pancreas, as well as the same range of action as all other drugs of
the group of examined compounds. Tolazamide is used for non-insulin-dependent diabetes
mellitus without expressed microvascular complications. | [Uses]
Tolazamide | [Definition]
ChEBI: An N-sulfonylurea that is 1-tosylurea in which a hydrogen attached to the nitrogen at position 3 is replaced by an azepan-1-yl group. A hypoglycemic agent, it is used for the treatment of type 2 diabetes mellitus. | [Manufacturing Process]
1-Nitrosohexamethyleneimine: A solution of 89.5 grams of
hexamethyleneimine, 75 ml of concentrated hydrochloric acid and 36 ml of
water was heated to 70°C on a steam bath. The solution was made acidic by
adding 5 ml of 2 N hydrochloric acid. While maintaining the reaction mixture
at 70° to 75°C, a solution of 67 grams of sodium nitrite in 95 ml of water was
added with stirring over a period of 1 hour. The mixture was then stirred at
70°C for 2 hours, and then cooled. The upper oily layer was separated and
the aqueous layer was then extracted with ether. The combined ether extract
and oil was dried over anhydrous magnesium sulfate and concentrated to
dryness. Upon distillation of the residue there was obtained 1-
nitrosohexamethyleneimine as a yellow oil, boiling at 136° to 138°C/34 mm.
1-Aminohexamethyleneimine: To a mixture of 15.18 grams of lithium
aluminum hydride and 400 ml of anhydrous ether was added about 10% of a
solution of 51.27 grams of 1-nitrosohexamethyleneimine in 100 ml of
anhydrous ether. The mixture was refluxed until the reaction started. The
remainder of the solution was added at such a rate as to maintain gentle
reflux. Refluxing was continued for 2 hours more, followed by the successive
addition of 16 ml of water, 12 ml of 20% aqueous sodium hydroxide solution
and 56 ml of water. The inorganic precipitate was removed by filtration and
washed with ether. The filtrate and ether washes were dried and the ether
was removed by evaporation. Upon distillation of the residue there was
obtained 25.46 grams (56%) of 1-aminohexamethyleneimine as a colorless
liquid boiling at 94° to 96°C/55 mm.
N-(4-Methylbenzenesulfonyl)-N'-Hexamethyleneiminourea Free Base: A
mixture of 11.42 grams of 1-aminohexamethyleneimine and 24.33 grams of
4-methylbenzenesulfonylurethane was heated at 130°C (oil-bath temperature)
for 2 hours. The resulting ethanol and unreacted amine were removed at 15
mm pressure for 2 hours while keeping the oil bath at 130°C. The residue was
cooled and recrystallized from methanol, giving 16.73 grams (54%) of N-(4-
methylbenzenesulfonyl)-N'-hexamethyleneiminourea free base melting at 163°
to 166°C. After a second recrystallization from methanol, the melting point
was 163.5° to 166.5°C. | [Therapeutic Function]
Oral hypoglycemic | [Synthesis]
Tolazamide is 1-hexahydro-1H-azepin-1-yl)-3-(p-toluenesulfonyl)urea
(26.2.8). By maintaining structural similarities with first-generation drugs, this drug differs
from the other drugs examined in that it has a semicarbazide group instead of a urea
residue, and an azepine group instead of a cyclohexyl group. It is synthesized by reacting with ethyl-(p-toluenesulfonyl)carbamate (26.2.7), which is made from p-toluenesulfonamide
and ethylchloroformate, with 1-aminoazepine.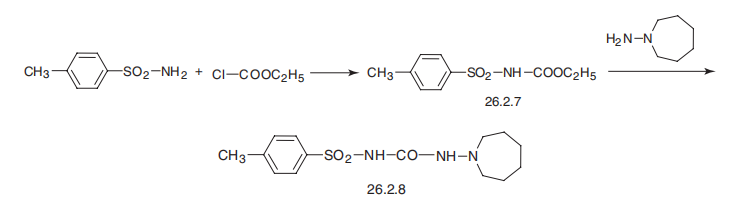
| [Toxics Screening Level]
The initial threshold screening level (ITSL) for tolazamide is 17 μg/m3 based on an annual averaging time. | [References]
[1] MURALI GOPALAKRISHNAN. Pharmacology of human sulphonylurea receptor SUR1 and inward rectifier K+ channel Kir6.2 combination expressed in HEK-293 cells[J]. British Journal of Pharmacology, 2009, 129 7: 1323-1332. DOI: 10.1038/sj.bjp.0703181
[2] P H WANG. Coordinate regulation of glucose transporter function, number, and gene expression by insulin and sulfonylureas in L6 rat skeletal muscle cells.[J]. Journal of Clinical Investigation, 1989, 84 1: 62-67. DOI: 10.1172/jci114170
[3] JASON I. BIEDERMAN. Effects of sulfonylureas, α-endosulfine counterparts, on glomerulosclerosis in type 1 and type 2 models of diabetes[J]. Kidney international, 2005, 67 2: Pages 554-565. DOI: 10.1111/j.1523-1755.2005.67112.x |
|
|