| Identification | More | [Name]
Birch-Me | [CAS]
119-36-8 | [Synonyms]
2-Carbomethoxyphenol
2-HYDROXYBENZOIC ACID METHYL ESTER
2-(methoxycarbonyl)phenol
analgit
BETULA OIL
BIRCH OIL
BIRCH OIL, SWEET
exagien
FEMA 2154
FEMA 2745
Flucarmit
Gaultheria oil
Linsal
METHYL 2-HYDROXYBENZOATE
METHYL HYDROXYBENZOATE
METHYLIS SALICYLAS
METHYL-O-HYDROXYBENZOATE
METHYL SALICYLATE
o-Hydroxybenzoic acid methyl ester
OIL OF WINTERGREEN | [EINECS(EC#)]
204-317-7 | [Molecular Formula]
C8H8O3 | [MDL Number]
MFCD00002214 | [Molecular Weight]
152.15 | [MOL File]
119-36-8.mol |
| Chemical Properties | Back Directory | [Appearance]
colourless liquid with an odour of wintergreen | [Melting point ]
-8 °C | [Boiling point ]
222 °C(lit.)
| [density ]
1.174 g/mL at 25 °C(lit.) | [vapor density ]
5.26 (vs air)
| [vapor pressure ]
1 mm Hg ( 54 °C)
| [FEMA ]
2745 | [refractive index ]
n20/D 1.536(lit.)
| [Fp ]
226 °F
| [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
Very slightly soluble in water, miscible with ethanol (96 per cent) and with fatty and essential oils. | [form ]
Liquid | [pka]
pKa 9.90 (Uncertain) | [color ]
Clear colorless to pale yellow | [Odor]
at 10.00 % in dipropylene glycol. wintergreen mint | [Stability:]
Stable. Incompatible with strong oxidizing agents, strong bases. | [biological source]
synthetic | [Odor Type]
minty | [Water Solubility ]
0.07 g/100 mL (20 ºC) | [JECFA Number]
899 | [Merck ]
14,6120 | [BRN ]
971516 | [Dielectric constant]
9.0(20℃) | [Major Application]
flavors and fragrances | [Cosmetics Ingredients Functions]
SOOTHING
PERFUMING
FLAVOURING
ORAL CARE
DENATURANT | [Cosmetic Ingredient Review (CIR)]
Methyl salicylate (119-36-8) | [InChI]
1S/C8H8O3/c1-11-8(10)6-4-2-3-5-7(6)9/h2-5,9H,1H3 | [Contact allergens]
This anti-inflammatory agent is found in a wide number of ointments and can induce allergic contact dermatitis. | [InChIKey]
OSWPMRLSEDHDFF-UHFFFAOYSA-N | [SMILES]
COC(=O)c1ccccc1O | [LogP]
2.55 | [Surface tension]
41.0 mN/m at 278.15K | [CAS DataBase Reference]
119-36-8(CAS DataBase Reference) | [NIST Chemistry Reference]
2-Hydroxybenzoic acid methyl ester(119-36-8) | [EPA Substance Registry System]
119-36-8(EPA Substance) |
| Hazard Information | Back Directory | [Chemical Properties]
colourless liquid with an odour of wintergreen | [Definition]
ChEBI: A benzoate ester that is the methyl ester of salicylic acid. | [General Description]
Colorless yellowish or reddish liquid with odor of wintergreen. | [Reactivity Profile]
METHYL SALICYLATE(119-36-8) is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. This chemical is incompatible with oxidizers. METHYL SALICYLATE(119-36-8) is also incompatible with strong bases. METHYL SALICYLATE(119-36-8) may react with iron salts. | [Air & Water Reactions]
Insoluble in water. | [Hazard]
Toxic by ingestion; use in foods restrictedby FDA, lethal dose 30 cc in adults, 10 cc in chil-dren. | [Health Hazard]
Harmful if swallowed, inhaled, absorbed through skin. Vapor mist is irritating to the eyes, mucous membranes, upper respiratory tract and skin. Ingestion of relatively small amount causes severe poisoning and death. Causes nausea, vomiting, acidosis, pulmonary edema, pneumonia, convulsions and death. | [Fire Hazard]
This chemical is combustible. | [Occurrence]
Numerous plants produce methyl salicylate in very small amounts. Some plants, such as the following, produce more:
Some species of the genus Gaultheria in the family Ericaceae, including Gaultheria procumbens, the wintergreen or eastern teaberry;
Some species of the genus Betula in the family Betulaceae, particularly those in the subgenus Betulenta such as B. lenta, the black birch;
All species of the genus Spiraea in the family Rosaceae, also called the meadowsweets.
This compound is produced most likely as an anti-herbivore defense. If the plant is infected with herbivorous insects, the release of methyl salicylate may function as an aid in the recruitment of beneficial insects to kill the herbivorous insects.Aside from its toxicity, methyl salicylate may also be used by plants as a pheromone to warn other plants of pathogens such as tobacco mosaic virus. | [Preparation]
Methyl salicylate can be produced by esterifying salicylic acid with methanol. Commercial methyl salicylate is now synthesized, but in the past, it was commonly distilled from the twigs of Betula lenta (sweet birch) and Gaultheria procumbens (eastern teaberry or winter green). | [Production Methods]
Methyl acetate, a novel acyl acceptor for biodiesel production has been developed, and a comparative study on Novozym 435-catalyzed transesterification of soybean oil for biodiesel production with different acyl acceptors has been studied (Noureddini et al., 2005).
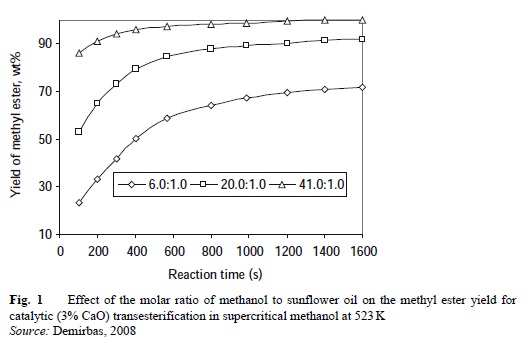
Figure 1 shows the effect of the molar ratio of methanol to sunflower oil on the methyl ester yield for catalytic (3% CaO) transesterification in supercritical methanol at 523 K. | [Composition]
The leaves of wintergreen are reported to contain arbutin, caffeic acid, ericolin, ferulic acid, gaultherase, gaultheric
acid, gaultherin, gentisinc acid, methyl salicylate (5445 to 7920 ppm) o-pyrocatachuic acid, p-coumaric acid, p-hydroxybenzoic acid,
primverose, protocatachuic acid, syringic acid, tannic acid, tannin, tricontane and vallininc acid. | [Aroma threshold values]
Detection: 40 ppb | [Taste threshold values]
Taste characteristics at 10 ppm: sweet, salicylate and root beer with aromatic and balsamic nuances | [Synthesis Reference(s)]
Canadian Journal of Chemistry, 61, p. 688, 1983 DOI: 10.1139/v83-127
The Journal of Organic Chemistry, 40, p. 3649, 1975 DOI: 10.1021/jo00913a007
Tetrahedron Letters, 37, p. 153, 1996 DOI: 10.1016/0040-4039(95)02120-5 | [Flammability and Explosibility]
Nonflammable | [Biochem/physiol Actions]
Methyl salicylate plays an important role in fruit ripening. It is known to attract natural enemies of herbivores. MeSA inhibits the activity of aminocyclopropane-1-carboxylic acid synthase (ACC synthase) and aminocyclopropane-1-carboxylic acid oxidase (ACC oxidase) in plums and tomatoes, it can inhibit fungal infections and reduce chilling injury symptoms in fruits like pomegranates. This oil of wintergreen is of great interest in the tobacco industry as a flavorant. It has counter irritant and anti-inflammatory effects. | [Safety]
In pure form, methyl salicylate is toxic, especially when taken internally. A single teaspoon (5ml) of methyl salicylate contains 7g of salicylate, which is equivalent to more than twenty- three 300 mg aspirin tablets. The lowest published lethal dose is 101 mg / kg body weight in adult humans , (or 7.07 grams for a 70 - kg adult). It has proven fatal to small children in doses as small as 4 ml.[6] A seventeen-year- old cross - country runner at Notre Dame Academy on Staten Island, died in April 2007, after her body absorbed methyl salicylate through excessive use of topical muscle-pain relief products.
Most instances of human toxicity due to methyl salicylate are a result of over-application of topical analgesics, especially involving children. Some people have intentionally ingested large amounts of oil of wintergreen. Salicylate, the major metabolite of methyl salicylate, may be quantitated in blood, plasma or serum to confirm a diagnosis of poisoning in hospitalized patients or to assist in an autopsy. | [Synthesis]
By esterification from natural sources; by esterification of salicylic acid with methanol | [Carcinogenicity]
Available data suggest that
methyl salicylate is not carcinogenic. | [Purification Methods]
Dilute the ester with Et2O, wash with saturated NaHCO3 (it may effervesce due to the presence of free acid), brine, dry MgSO4, filter, evaporate and distil it. Its solubility is 1g/1.5L of H2O. The benzoyl derivative has m 92o (b 270-280o/120mm), and the 3,5-dinitrobenzoate has m 107.5o, and the 3,5-dinitrocarbamoyl derivative has m 180-181o. [Hallas J Chem Soc 5770 1965, Beilstein 10 IV 143.] | [Toxicity evaluation]
Oral LD50 values (mg/kg) for mouse, rat and rabbit are 1110, 887 and 1300, respectively. Oral LD50 values for child and adult human (mg/kg) are 228 and 506, respectively. Topical methyl salicylate generally rarely causes systemic toxicity, but it can be absorbed by the skin leading to stimulation of the central nervous system respiratory center, disturbance of lipid and carbohydrate metabolism, and disturbance of intracellular respiration. Severe poisoning can lead to acute lung injury, lethargy, coma, seizures, cerebral edema, and death. In the case of salicylate intoxication, treatment includes general supportive care, purification of the gastrointestinal tract with activated charcoal in the case of ingestion of salicylic acid, and monitoring of serum salicylic acid concentration. Bicarbonate infusion or hemodialysis can be used to enhance the elimination of salicylic acid. |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R36/38:Irritating to eyes and skin .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S24/25:Avoid contact with skin and eyes . | [RIDADR ]
3082 | [WGK Germany ]
1
| [RTECS ]
VO4725000
| [F ]
8 | [Autoignition Temperature]
847 °F | [TSCA ]
Yes | [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
29182300 | [Storage Class]
10 - Combustible liquids | [Hazard Classifications]
Acute Tox. 4 Oral
Aquatic Chronic 3
Eye Dam. 1
Repr. 2
Skin Sens. 1B | [Safety Profile]
Human poison by
ingestion. Moderately toxic to humans by an
unspecified route. Moderately toxic
experimentally by intraperitoneal,
intravenous, and subcutaneous routes. An
experimental teratogen. Human systemic
effects by ingestion: flaccid paralysis without
anesthesia, general anesthesia, dyspnea,
nausea, vomiting, and respiratory
stimulation. Experimental reproductive
effects. A severe skin and eye irritant.
Ingestion of relatively small amounts has
caused severe poisoning and death.
Combustible liquid when exposed to heat or
flame; can react with oxibzing materials. To
fight fire, use CO2, dry chemical. When
heated to decomposition it emits acrid
smoke and irritating fumes. | [Hazardous Substances Data]
119-36-8(Hazardous Substances Data) | [Toxicity]
LD50 orally in rats: 887 mg/kg (Jenner) |
| Questions And Answer | Back Directory | [description]
Natural material is found in wintergreen oil, oil of wintergreen, birch oil, green tea seed oil, clove oil, Quercetin tree oil, tuberose oil, small when medicated oil, tea oil, ylang ylang oil and cherry, apple, strawberry fruit juice. It is colorless or pale yellow, red or light yellow transparent oily liquid. A strong aroma of wintergreen oil. Relative molecular mass of 152.16. The relative density of 1.1738. Melting point-8.6 ℃. Boiling point 223.3 ℃. A flash point of 96 ℃. The refractive index of 1.5360. It is insoluble in water, soluble in alcohol, ether, acetic acid and other common organic solvents. It is easy to change when exposed in air . Rat oral LD50887mg/kg.
Salicylic acid and methanol are heated to prepare it in the presence of sulfuric acid . Or it is made of stone STSP plant holly leaf through distillation.
Methyl salicylate can be used in the formulation of flavors, mainly for the deployment of ylang ylang, tuberose, chypre, acacia, fougere, orchids. But the most common use is perfuming toothpaste. IFRA has no restrictions.
Methyl salicylate is recognized as GRAS by FEMA, FEMA number 2745, the European Council includes methyl salicylate in health artificial flavorants table which can be used in food without harm to human , the maximum amount is 8400mg/kg, ADI value is 0.5mg/kg.
Methyl salicylate can be used for food flavor formula, is a food flavor within China's GB2760-1996 provisions of the temporary use , ADI value of 0 to 0.5, is commonly used in the deployment of strawberry, vanilla, grapes and other fruit-flavor flavor for food and beer, but also flavor fructose, soft drinks.Methyl salicylate is also used as preservatives, pesticides, polishes, inks, paints, polyester dye carriers in industrial fields
| [application]
Methyl salicylate ointment is a common dermatology drug with analgesic, anti-inflammatory, bactericidal effect. Because it can induce local irritation, it is rarely orally used.When spread in the skin,it is easily absorbed. Its liniments, ointments can be used for acute rheumatoid arthritis, low back pain and muscle pain, also itching and flavoring agents, flavoring agents, preservatives.
| [Uses]
Appropriately use it as modifiers for some floral types , such as ylang ylang, magnolia, acacia, shy flower, tuberose, gardenia, bloom, sweet clover grass.It is mainly used for medicine and hygiene products such as toothpaste, tooth powder, mouthwash, talcum powder, carminative oil. Methyl salicylate can also be used for industrial products such as glue, glue paper, card paste, paste. It can also be used for food flavor,such as strawberries, grapes, walnuts, spearmint, spicy flavor as a special green gas.
Methyl salicylate is used as high-temperature heat carrier in dyeing industry .
The product has similar-Pyrola odor ,when used as a spice, it is often used as flavoring agent for pharmaceutical drugs and the pigmentum.It also has applications in blending spices, such as Quercetin trees in general flavors. Methyl salicylate is also used as solvents and intermediates for the manufacture of pesticides, fungicides, perfumes, paints, cosmetics, ink and dye fibers such help. Methyl salicylate reacts with ammonia to make salicylamide which is used for production of antipyretic analgesics salicylaldehyde ethyl amine ,and salicylamide itself is an anti-inflammatory drug. Methyl salicylate can be obtained by acetylation using acetylsalicylic acid methyl ester (C10H10O4, [580-02-9]). Add methyl salicylate and acetic anhydride into pot ,put sulfuric acid under stirring, the reaction temperature does not exceed 60 ℃ for about 1h, the reaction is completed, the reaction is poured into ice water and precipitated crystals are filtered, washed, dried, and acetylsalicylic acid methyl ester is obtained. Acetylsalicylic acid can be prepared by cyclization to get 4-hydroxy coumarin. Methyl salicylate is used as a pharmaceutical for external application agent.
Perfumes and suntan lotion ultraviolet absorber.
| [Production Method]
Methyl salicylate is widespread in nature, and it is a main ingredient of wintergreen, small medicated oil . Alsoit is present in essential oils of the tuberose, Quercetin tree, ylang ylang, cloves, tea. Salicylic acid and methanol are used to make it in the presence of sulfuric acid through esterification. Salicylic acid is dissolved in methanol, add sulfuric acid, heat with stirring, the reaction time is 3h,90-100℃, cool to below 30 ℃,take oil ,wash with sodium carbonate solution to pH8 above, and then wash 1 time with water. Vacuum distillation, collect 95-110 ℃ (1.33-2.0kPa) distillate, obtain methyl salicylate. The yield is over 80%. General industrial methyl salicylate content is 99.5%. Material consumption fixed: Acid 950kg/t, methanol 400kg/t.
|
|
|