| Identification | Back Directory | [Name]
Di-chlorobis[(1,2,3-)-1-phenyl-2-propenyl]dipalladium(II), 98% | [CAS]
12131-44-1 | [Synonyms]
Cinnamylpalladium chloride dimer
BIS[CINNAMYL PALLADIUM(II) CHLORIDE]
PalladiuM(II)(π-cinnaMyl) Chloride DiMer
PalladiuM(pi-cinnaMyl) chloride diMer 97%
PalladiuM(II)(pi-cinnaMyl) Chloride DiMer
Di-μ-chlorobis[(1,2,3-η)-1-phenyl-2-propenyl]dipalladium(Ⅱ)
Di-μ-chlorobis[(1,2,3-η)-1-phenyl-2-propen-1-yl]dipalladium
Di-chlorobis[(1,2,3-)-1-phenyl-2-propenyl]dipalladium(II), 98%
Di-μ-chlorobis[(1,2,3-η)-1-phenyl-2-propenyl]dipalladiuM(II),98% | [EINECS(EC#)]
234-579-8 | [Molecular Formula]
[(C9H9)ClPd]2 | [MDL Number]
MFCD16621470 | [MOL File]
12131-44-1.mol | [Molecular Weight]
512.03 |
| Chemical Properties | Back Directory | [Melting point ]
218-220°C | [storage temp. ]
Inert atmosphere,2-8°C | [form ]
crystal | [color ]
yellow | [Sensitive ]
air sensitive | [InChI]
InChI=1S/2C9H6.2ClH.2Pd/c2*1-2-6-9-7-4-3-5-8-9;;;;/h2*1,3-5,7-8H;2*1H;;/q2*-1;;;2*+2/p-2 | [InChIKey]
BCEGUOGHFLWSPJ-UHFFFAOYSA-L | [SMILES]
[Pd+2]123(C=C1[C-]2C1C=CC=CC=1)[Cl-][Pd+2]12(C=C1[C-]2C1C=CC=CC=1)[Cl-]3 |
| Questions And Answer | Back Directory | [Application]
Di-chlorobis[(1,2,3-)-1-phenyl-2-propenyl]dipalladium(ii) is an important organic regant for the use of transition-metal-mediated organic syntheses. It can be used as a catalyst for the ammonia cross-coupling reactions to synthesize arylamines and conversion of aryl triflates to fluorides.
| [Reaction]
- Precursor for the preparation of a palladium catalyst used in the carbonylative arylation of ketones, yielding vinylbenzoate compounds.
- Precursor for the preparation of a palladium catalyst used in the Buchwald-Hartwig amination of (hetero)aryl chlorides.
- Precursor for the preparation of a palladium catalyst used in the arylative dearomatization of phenols.
- Versatile palladium precursor for the preparation of palladium catalysts used in the cross-coupling of aryl chlorides and amines , conversion of aryl triflates to aryl fluorides , and the α-arylation of aldehydes .
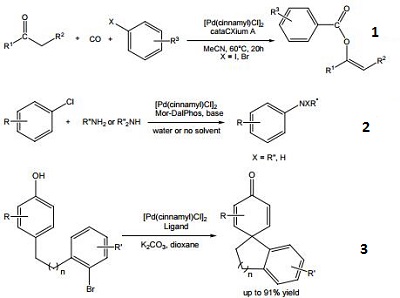
|
| Hazard Information | Back Directory | [Uses]
Palladium(π-cinnamyl) chloride dimer can be used as a catalyst for the:
- Ammonia cross-coupling reactions to synthesize arylamines.
- Conversion of aryl triflates to fluorides.
It can also be used as a source of palladium in the asymmetric α-arylation of amides. | [reaction suitability]
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst | [Synthesis]
A) In a double-necked flask equipped with magnetic stirring, 250 mL of distilled water was added and passed under argon gas for 30 min; B) Continuing to keep under the flow of passed argon gas, PdCl2 (10 mmol,1.77 g,1 equiv) and KCl (20 mmol,1.42 |
|
|