| Identification | Back Directory | [Name]
Pixantrone | [CAS]
144510-96-3 | [Synonyms]
CS-1359
pixantrone
Pixantrone Dimaleate
Pixantrone, BBR 2778
BBR-2778;BBR2778;BBR 2778
Pixantrone diformate salt
6,9-Bis[(2-aminoethyl)amino]benz[g]isoquinoline-5,10-dione
6,9-bis((2-aminoethyl)amino)benzo(g)isoquinoline-5,10-dione
Benz[g]isoquinoline-5,10-dione, 6,9-bis[(2-aminoethyl)amino]- | [Molecular Formula]
C17H19N5O2 | [MDL Number]
MFCD09837694 | [MOL File]
144510-96-3.mol | [Molecular Weight]
325.37 |
| Chemical Properties | Back Directory | [Melting point ]
>173°C (dec.) | [density ]
1.405 | [storage temp. ]
Refrigerator | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [color ]
Dark Blue to Very Dark Blue |
| Hazard Information | Back Directory | [Description]
In May 2012, pixantrone was approved by the European Commission as a single agent for the treatment of relapsed or refractory aggressive B-cell non-Hodgkin lymphoma (NHL) in adult patients who have failed on at least two previous therapies. Pixantrone (also known as BBR- 2778) is an anthracycline analogue that was specifically designed to address the cardiotoxicity seen in earlier agents by replacement of a 1,4- dihydroxyanthracene-9,10-dione core with a benzoisoquinoline-5,10-dione ring system. Pixantrone inhibits topoisomerase II by intercalation with DNA and is also believed to form covalent adducts with the N-2 amino group of guanine via a formaldehyde aminal formed with the primary amino groups. Pixantrone is less cytotoxic than other anthracycline derivatives, but shows good antitumor activity in vivo in a variety of preclinical tumor models, including leukemia and lymphoma models. Pixantrone also demonstrated significantly reduced cardiotoxicity in preclinical models compared with the anthracyclines doxorubicin and mitroxantrone. Pixantrone was synthesized by Friedel–Crafts reaction of pyridine-3,4-dicarboxylic acid anhydride with 1,4-difluorobenzene to give a ketoacid that was cyclized to the tricyclic core by treatment with fuming sulfuric acid at 140℃. Reaction of the resulting 6,9-difluorobenzoisoquinoline-5-10-dione with ethylenediamine followed by careful pH adjustment and treatment with maleic acid gave pixantrone in good overall yield and purity. | [Originator]
University of Vermont (United States) | [Uses]
anti-inflammatory and immunosuppressive glucocorticoid steroid | [Uses]
Pixantrone is an antineoplastic drug belonging to group of antitumor antibiotics. Pixantrone is an anlogue of Mitoxantrone (M373425) and is just as potent in the treatment of multiple sclerosis with fewer toxic effects on cardiac tissue. Studies suggest that Pixantrone significantly reduces amyloid beta (A beta(1-42)) neurotoxicity, a mechanism implicated in Alzheimer's disease. | [Definition]
ChEBI: Pixantrone is a member of isoquinolines. | [Brand name]
Pixuvri | [Clinical Use]
Pixuvri (Pixantrone dimaleate) is a novel aza-anthracenedione derivative approved in Europe for the
treatment of adult patients with non-Hodgkin B-cell lymphoma. It is also being pursued as a
treatment for various cancers, and specifically as an alternative to other structurally-related drugs like
mitoxantrone, employed for treatment of breast cancer, acute myeloid leukemia (AML), and non-
Hodgkins lymphoma.Pixantrone dimaleate has been designed to maintain antitumor efficacy while
decreasing highly cardiotoxic side effects observed during treatment with other related anti-tumor
anthracenedione derivatives. Like many anthracenedione drugs, the mechanism of action for pixantrone dimaleate likely includes a number of pathways and processes, with studies suggesting
intercalation into DNA and/or interference with DNA –Topoisomerase II activity, leading to subsequent
protein associated-DNA strand breaks and eventually to cell death. | [Synthesis]
Pixantrone dimaleate, also
known as BBR 2778, was originally synthesized by professors Krapcho and Hacker at the University of
Vermont, and determination of in-vitro tumor cell cytotoxicity was co-identified by the Boehringer
Mannheim Italia research center and the University of Vermont. After the merger of Boehringer
Mannheim with La-Roche, Novuspharm, and Cell Therapeautics, Inc., pixantrone dimaleate has been
developed and marketed by Cell Therapeutics, Inc.
The manufacturing scale synthesis of pixantrone dimaleate relies on several process modifications, from the original synthesis reported by Krapcho in 1994.169 This modified procedure has provided
active pharmaceutical ingredient (API) in high purity (>99%) and is acceptable for use in
pharmaceutical applications (the scheme). Beginning with pyridine 3,4-dicarboxylic acid (129),
generation of the corresponding anhydride 130 proceeded in 76% yield upon treatment with refluxing
Ac2O. Next, an AlCl3-promoted Friedel-Crafts reaction of 1,4-difluorobenzene (131) with 130 under
reflux conditions provided a mixture of nicotinic acid isomers 132a/132b in 84% yield, which were
carried directly to the next step. Cyclization with fuming H2SO4 yielded the desired difluorobenzoisoquinoline-
dione core 133, which was further functionalized with ethylenediamine (134) to provide
the free base of pixantrone. Subjection of the pixantrone free base to aqueous acetic anhydride and
maleic acid provided pixantrone dimaleate (XX) in 92% yield over 3 steps.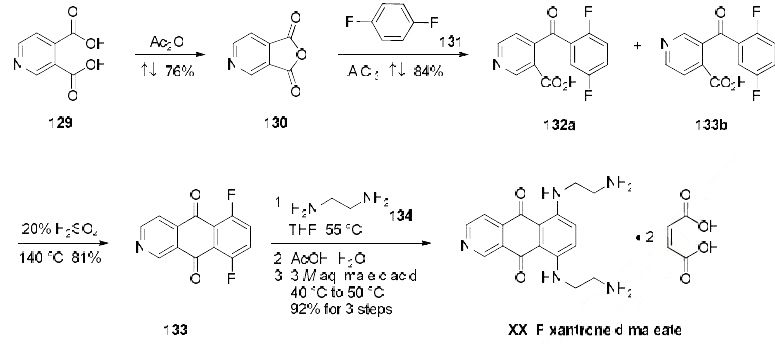
| [Drug interactions]
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Live vaccines: risk of generalised infections - avoid. | [Metabolism]
Pixantrone may be metabolised in the liver and/or
excreted in the bile. As metabolism appears to be limited,
biliary excretion of unchanged pixantrone may be the
major elimination pathway. Acetylated metabolites were
pharmacologically inactive and metabolically stable.
In human urine, the compound was mainly excreted
unchanged, and very small amounts of phase I and phase
II acetylated metabolites were found. |
|
|