| Identification | More | [Name]
Sivelestat sodium | [CAS]
201677-61-4 | [Synonyms]
n-[2-[[[4-(2,2-dimethyl-1-oxopropoxy)phenyl]sulfonyl]amino]benzoyl]-(s)-glycine monosodium salt tetrahydrate
SIVELESTAT SODIUM HYDRATE
SIVELESTAT SODIUM TETRAHYDRATE
Sivelestat(anhydrous)
N-[2-[[[4-(2,2-Dimethyl-1-oxopropoxy)phenyl]sulfonyl]amino]benzoyl]-(S)-glycine monosodium salt tetrahydrate | [Molecular Formula]
C20H29N2NaO11S | [MDL Number]
MFCD01937969 | [Molecular Weight]
528.51 | [MOL File]
201677-61-4.mol |
| Chemical Properties | Back Directory | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Soluble in DMSO (up to 20 mg/ml). | [form ]
White solid. | [color ]
White | [Stability:]
Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 2 months | [InChIKey]
YMOIFFHCERRYCO-UHFFFAOYSA-N | [SMILES]
C(C1C=CC=CC=1NS(C1C=CC(OC(=O)C(C)(C)C)=CC=1)(=O)=O)(=O)NCC(=O)O.[NaH].O | [CAS DataBase Reference]
201677-61-4(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Description]
Sivelestat is an acyl enzyme inhibitor of neutrophil elastase, developed as an injectable
formulation for the treatment of acute lung injury associated with systemic inflammatory
response syndrome. A neutrophil predominant inflammation associated with excessive
release of human neutrophil elastase (HNE) from azurophilic granules is capable of
damaging both the lung parenchymal cells and the extracellular matrix allowing an alveolar
capillary barrier disruption. Sivelestat is a sulfonanilide-containing pivaloyloxy benzene
derivative prepared in a three step synthesis. This agent acts as a reversible and selective
inhibitor of HNE with an lG.0 value of 0.044 PM. Sivelestat has exhibited potent protective
effects against various causes of lung injuries in animal models. In an acid-induced acute
lung injury model in conscious hamster, administration of sivelestat for 48 h following HCI
instillation, dose-dependently reduced mortality and significantly improved the protein
levels in bronchoalveolar lavage fluids and pulmonary artery pressure. In a similar study,
the agent inhibited the endotoxin-induced acute lung dysfunctions (marked elevation of
pulmonary vascular permeability, leukocyte migration, hemorrhage and parenchymal
injury) in different animal species. Moreover, in a cardiopulmonary bypass model in dog,
sivelestat ameliorated the respiratory index and interstitial-intra-alveolar edema. Sivelestat
has a relatively poor bioavailability, due to an extensive first-pass metabolism and is easily
hydrolyzed in vitro to an inactive metabolite. In two animal species, the half-life time was
approximately 5-7 min. Clinical trials have shown that treatment with the agent improves
respiratory function and facilitates early removal of patients from mechanical ventilation.
However, in the last clinical study, conducted by Eli Lilly in patients with acute lung injury,
no difference in mortality and safety was seen between sivelestat and placebo. | [Originator]
Ono Pharmaceutical (Japan) | [Uses]
Treatment of acute lung injury; acute respiratory distress syndrome (elastase inhibitor). | [Definition]
ChEBI: Sivelestat sodium hydrate is a N-acylglycine. It is functionally related to a N-benzoylglycine. | [Brand name]
Elaspol | [Synthesis]
The synthesis of
sivelestat (24) started with the amide formation between 2-
nitrobenzoyl chloride (203) and glycine benzyl ester p-tolene
sulfonic acid salt (204) in the presence of TEA to give amide
205 in 90% yield. Amide 205 was then reduced with iron power under acidic conditions to give corresponding amine
206 in 81% yield. Alternatively, the mixture of activated
Raney nickel, nitro compound 205, acetic acid and 1,3-
dimethyl-2-imidazolinone (DMI) under 25 atmospheric
pressure of hydrogen at 40oC in an autoclave can give the
same free amine 206 in 88% yield. Free amine 206 was
treated with p-pivaloyloxybenzenesulfonyl chloride
(207) in pyridine to yield sulfonamide 208 in 87% yield.
Benzyl ester 208 was converted to its free carboxylic acid
under hydrogenation, and the carboxylic acid was
subsequently basified to give sivelestat sodium (24).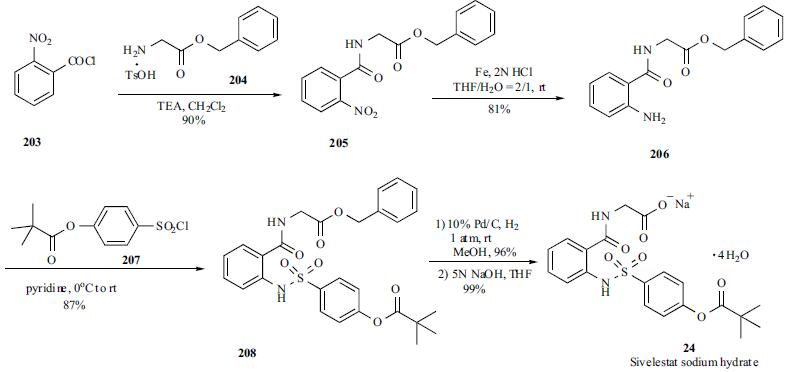
| [References]
1) Kawabata et al. (1991), ONO-5046, a novel inhibitor of human neutrophil elastase; Biochem. Biophys. Res. Commun., 177 814
2) Hagiwara et al. (2009), Neutrophil elastase inhibitor (sivelestat) reduces the levels of inflammatory mediators by inhibiting NFκB; Inflamm. Res., 58 198
3) Young et al. (2007), Role of neutrophil elastase in LTB4-induced neutrophil transmigration in vivo assessed with a specific inhibitor and neutrophil elastase deficient mice; Br. J. Phamracol., 151 628
4) Iwamoto et al. (2009), Protective effect of sivelestat sodium hydrate (ONO-5046) on ischemic spinal cord injury; Interact. Cardiovasc. Thorac. Surg., 8 606
5) Iwata et al. (2010), Effect of neutrophil elastase inhibitor (sivelestat sodium) in the treatment of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): a systemic review and meta-analysis; Intern. Med., 49 2423 |
|
|