| Identification | More | [Name]
CINOXACIN | [CAS]
28657-80-9 | [Synonyms]
1-ETHYL-1,4-DIHYDRO-4-OXO[1,3]DIOXOLO[4,5-G]CINNOLINE-3-CARBOXYLIC ACID
CINOXACIN
TIMTEC-BB SBB003082
1-ethyl-6,7-methylenedioxy-4(1h)-oxocinnoline-3-carboxylicacid
3)dioxolo(4,5-g)cinnoline-3-carboxylicacid,1,4-dihydro-1-ethyl-4-oxo-(
cinobac
1-ethyl-4-oxo-1,4-dihydro-[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid | [EINECS(EC#)]
249-133-8 | [Molecular Formula]
C12H10N2O5 | [MDL Number]
MFCD00056776 | [Molecular Weight]
262.22 | [MOL File]
28657-80-9.mol |
| Chemical Properties | Back Directory | [Melting point ]
261-262° (dec) | [Boiling point ]
405.47°C (rough estimate) | [density ]
1.3545 (rough estimate) | [refractive index ]
1.6660 (estimate) | [storage temp. ]
2-8°C | [solubility ]
1 M NaOH: soluble50mg/mL | [form ]
solid | [pka]
pKa 5.38(H2O t=25.0 I=0.025) (Uncertain) | [color ]
White to Off-White | [Stability:]
Hygroscopic | [InChI]
1S/C12H10N2O5/c1-2-14-7-4-9-8(18-5-19-9)3-6(7)11(15)10(13-14)12(16)17/h3-4H,2,5H2,1H3,(H,16,17) | [InChIKey]
VDUWPHTZYNWKRN-UHFFFAOYSA-N | [SMILES]
CCN1N=C(C(O)=O)C(=O)c2cc3OCOc3cc12 | [CAS DataBase Reference]
28657-80-9(CAS DataBase Reference) |
| Safety Data | Back Directory | [WGK Germany ]
2 | [RTECS ]
JI4640000 | [Storage Class]
11 - Combustible Solids | [Toxicity]
LD50 in rats (mg/kg): 4160 orally; 900 i.v. (Narama) |
| Hazard Information | Back Directory | [Originator]
Cinobac,Lilly,UK,1979 | [Uses]
antibacterial | [Uses]
Cinoxacin is an antibacterial quinolone previously known for its use in the treatment of urinary tract infections. | [Definition]
ChEBI: A member of the class of cinnolines that is 6,7-methylenedioxycinnolin-4(1H)-one bearing an ethyl group at position 1 and a carboxylic acid group at position 3. An analogue of oxolinic acid, it has similar antibacterial actions. It was
formerly used for the treatment of urinary tract infections. | [Manufacturing Process]
About 23 g (0.095 mol) of 1-ethyl-6,7-methylenedioxy-4(1H)-oxocinnoline-3-
carbonitrile were added to a mixture of 200 ml of concentrated hydrochloric
acid and 200 ml of acetic acid. The resultant reaction mixture was heated
under reflux for 18 hours, The excess acids were removed under vacuum, and
the residue was taken up in 150 ml of a 5% sodium bicarbonate solution. The
resultant solution was treated with 5 g of charcoal and filtered. The filtrate
was made acidic by the addition of hydrochloric acid and the resulting
precipitate was removed by filtration. 23 g, representing a yield of 91.6% of
1-ethyl-6,7-methylenedioxy-4(1H)-oxocinnoline-3-carboxylic acid as light tan
crystals which melted at 261°C to 262°C with decomposition were recovered. | [Brand name]
Cinobac
(Lilly). | [Therapeutic Function]
Antibacterial | [Antimicrobial activity]
This drug is effective with respect to Gram-negative microorganisms and is used for the
same indications as nalidixic and oxolinic acids. Synonyms of this drug are cinobactin,
nossacin, uronorm, and others. | [Pharmaceutical Applications]
A cinnoline derivative formulated for oral administration. It is active against most Enterobacteriaceae, but Ps. aeruginosa, Gram-positive bacteria and anaerobes are resistant.
It is well-absorbed when given orally. Administration with food reduces the peak concentration by about one-third, but the area under the concentration–time curve (AUC) remains unchanged. Concentrations in prostatic and bladder tissues reach 60% and 80%, respectively, of the simultaneous serum concentrations.
It is almost entirely eliminated in the urine, about 40–60% as unchanged drug and the rest as metabolites, most of which have no antibacterial activity. Urinary concentrations of active drug in the first 2 h after administration of a dose is 100–500 mg/L. Elimination is reduced by probenecid and by renal impairment, the half-life rising to about 12 h in endstage renal failure.
Adverse reactions that are common to the group are reported in 4–5% of patients; these are primarily gastrointestinal tract disturbances, but rashes occur in up to 3% and CNS disturbances in less than 1%. Use is restricted to uncomplicated urinary tract infection. | [Biological Activity]
Cinoxacin is a synthetic antimicrobial agent th at interferes with DNA replication by inhibiting DNA gyrase and topoisomerase IV (topo IV) through tight DNA binding. The mechanism of action of cinoxacin is comparable to nalidixic acid. Cinoxacin is effective against Gram-negative bacteria and is often used to tre at urinary tract infections caused by E. coliProteus mirabilisProteus vulgaris and Klebsiella sp. | [Clinical Use]
1-Ethyl-1,4-dihydro-4-oxo[1,3]dioxolo[4,5g]cinnoline-3-carboxylic acid (Cinobac) is a close congener (isostere) ofoxolinic acid (no longer marketed in the United States) andhas antibacterial properties similar to those of nalidixic andoxolinic acids.
It is recommended for the treatment of urinary tract infectionscaused by strains of Gram-negative bacteria susceptibleto these agents. Early clinical studies indicate that thedrug possesses pharmacokinetic properties superior to thoseof either of its predecessors. Thus, following oral administration,higher urinary concentrations of cinoxacin thanof nalidixic acid or oxolinic acid are achieved. Cinoxacinappears to be more completely absorbed and less proteinbound than nalidixic acid. | [Synthesis]
Cinoxacin, 1-ethyl-1,4-dihydro-4-oxo[1,3]-dioxolo[4,5-g]
cinnolin-3-carboxylic acid (33.2.14), is synthesized by a different scheme starting with
2-amino-4,5-methylendioxyacetophenone (33.2.10), which is synthesized by reducing
4,5-methylendioxy-2-nitroacetophenone with hydrogen over a platinum catalyst. In diazo�tation conditions, this undergoes spontaneous heterocyclization to 4-hydroxy-6,
7-methylendioxycinnoline (33.2.11) obviously due to the presence of a significant amount
of the enol form of acetophenone (33.2.10) under the reaction conditions. The resulting
cinnoline (33.2.11) then undergoes bromination by molecular bromine in the presence of
potassium acetate, giving 3-bromo-4-hydroxy-6,7-methylendioxycinnoline (32.2.12).
Upon reacting this with univalent copper cyanide in dimethylformamide, the bromine atom is replaced with a cyano group, forming the 3-cyano-4-hydroxy-6,7-methylen�dioxycinnoline (33.2.13). The resulting product is alkylated at the first position by ethyl
iodide using sodium hydride as a base, and the cyano group is hydrolyzed to a carboxyl
group using a mixture of hydrochloric and acetic acids, giving the desired cinoxacin.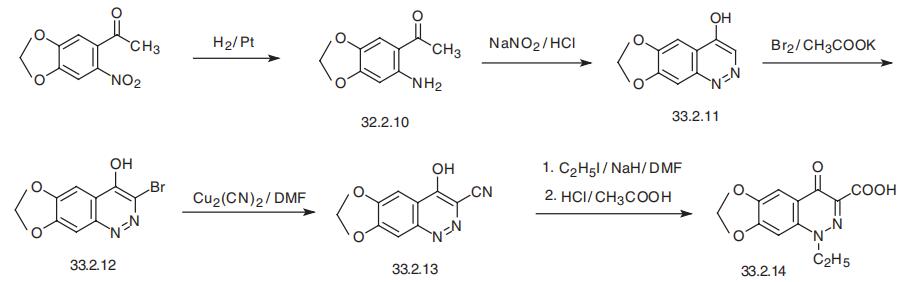
| [storage]
Store at -20°C |
|
|