| Identification | Back Directory | [Name]
2-Oxazolidinone, 5-(2,2-diMethyl-4H-1,3-benzodioxin-6-yl)-, (5R)- | [CAS]
452339-73-0 | [Synonyms]
BNKY020-VT04
Vilanterol Impurity 29
Vilanterol internate 1
(5R)-2-Oxazolidinone, 5-(2,2-diMethyl-4H-1,3-benzodioxin-6-yl)
(R)-5-(2,2-DIMETHYL-4H-BENZO[D][1,3]DIOXIN-6-YL)OXAZOLIDIN-2-ONE
2-Oxazolidinone, 5-(2,2-diMethyl-4H-1,3-benzodioxin-6-yl)-, (5R)-
(R)-5-(2,2-Dimethyl-4H-1,3-benzodioxin-6-yl)-1,3-oxazolidin-2-one
(5R)-5-(2,2-DiMethyl-4H-1,3-benzodioxin-6-yl)-1,3-oxazolidin-2-one | [EINECS(EC#)]
1592732-453-0 | [Molecular Formula]
C13H15NO4 | [MDL Number]
MFCD21362968 | [MOL File]
452339-73-0.mol | [Molecular Weight]
249.26 |
| Chemical Properties | Back Directory | [Melting point ]
160 - 163°C | [Boiling point ]
489.6±45.0 °C(Predicted) | [density ]
1.223±0.06 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,Store in freezer, under -20°C | [solubility ]
Chloroform (Sparingly), Methanol (Slightly) | [form ]
Solid | [pka]
12.29±0.40(Predicted) | [color ]
White to Off-White | [InChI]
InChI=1S/C13H15NO4/c1-13(2)16-7-9-5-8(3-4-10(9)18-13)11-6-14-12(15)17-11/h3-5,11H,6-7H2,1-2H3,(H,14,15)/t11-/m0/s1 | [InChIKey]
JUEBDVANOFZMMX-NSHDSACASA-N | [SMILES]
O1[C@H](C2=CC=C3OC(C)(C)OCC3=C2)CNC1=O |
| Hazard Information | Back Directory | [Uses]
(5R)-5-(2,2-diMethyl-4H-1,3-benzodioxin-6-yl)-2-Oxazolidinone is a reagent applied in the preparation of alkyl-linked di-phenyl aminoalcohols as long-acting β2 adrenergic receptor agonist. It is used as an antedrug.
| [Synthesis]
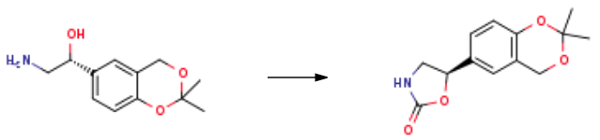
(R)-2-amino-1-(2,2-dimethyl-4H-benzo[d][1,3]dioxin-6-yl)ethan-1-ol (29.8g, 134.0mmol, 1.0eq.) and tetrahydrofuran (300mL) were added to the reaction flask under nitrogen protection, the reaction solution was heated to 40-60°C, and N, N'- Carbonyldiimidazole (22.7 g, 140.0 mmol, 1.05 eq.).After the addition, the reaction solution was kept at a temperature of 40-60° C. and stirred until the raw material reaction was completed. After the reaction, the solution was cooled to 15-30° C., and water (400 mL) was added. Then, the mixture was continuously cooled to 0-10°C and kept stirring. The precipitated solid was filtered, and the filter cake was washed with water. The filter cake was collected and dried under reduced pressure to obtain 2-Oxazolidinone, 5-(2,2-diMethyl-4H-1,3-benzodioxin-6-yl)-, (5R)- (30 g, 120.4 mmol, 89.9% yield).
| [References]
[1] Patent: CN106957313, 2017, A. Location in patent: Paragraph 0014-0015
[2] Organic and biomolecular chemistry, 2003, vol. 1, # 7, p. 1106 - 1111
[3] Patent: US2015/239862, 2015, A1. Location in patent: Paragraph 0133; 0134
[4] Patent: WO2004/22547, 2004, A1. Location in patent: Page 43-44
[5] Patent: WO2004/37773, 2004, A1. Location in patent: Page 46 |
|
|