| Identification | More | [Name]
Mecamylamine hydrochloride | [CAS]
826-39-1 | [Synonyms]
2-[METHYLAMINO]ISOCAMPHANE HYDROCHLORIDE
inversine
MECAMYLAMINE
MECAMYLAMINE HYDROCHLORIDE
N,2,3,3-TETRAMETHYLBICYCLO[2.2.1]HEPTAN-2-AMINE HYDROCHLORIDE
3-methylaminoisocamphanehydrochloride
3-methylaminoisokamfanchlorid
inversinehydrochloride
mecaminehydrochloride
mekaminhydrochloride
mevasinhydrochloride
n,2,3,3-tetramethyl-2-norbornanaminehydrochloride
n,2,3,3-tetramethyl-2-norbornanaminhydrochloride
n-methyl-dl-isobornylaminehydrochloride
Mecamylamine HCl
MECAMYLAMINE USP
MecamycamineHydrochloride
Bicyclo2.2.1heptan-2-amine, N,2,3,3-tetramethyl-, hydrochloride
2-NORBORNANAMINE,N,2,3,3-TETRAMETHYL-,HYDROCHLORIDE
2-(Methylamino)isocamphane hydrochloride, Inversine, N,2,3,3-Tetramethylbicyclo[2.2.1]heptan-2-amine hydrochloride | [EINECS(EC#)]
212-555-8 | [Molecular Formula]
C11H22ClN | [MDL Number]
MFCD00151462 | [Molecular Weight]
203.75 | [MOL File]
826-39-1.mol |
| Chemical Properties | Back Directory | [Melting point ]
>240°C (dec.) | [storage temp. ]
2-8°C | [solubility ]
ethanol: 122 mg/mL
| [form ]
neat | [pka]
pKa 11.2(H2O,t undefined,I=0.1) (Uncertain) | [color ]
White to off-white | [Water Solubility ]
H2O: 100mM | [Stability:]
Hygroscopic | [InChI]
InChI=1/C11H21N.ClH/c1-10(2)8-5-6-9(7-8)11(10,3)12-4;/h8-9,12H,5-7H2,1-4H3;1H/t8-,9+,11?;/s3 | [InChIKey]
PKVZBNCYEICAQP-YENZGJFJNA-N | [SMILES]
C1(C)(NC)C(C)(C)[C@]2([H])CC[C@@]1([H])C2.Cl |&1:7,11,r| | [CAS DataBase Reference]
826-39-1(CAS DataBase Reference) | [EPA Substance Registry System]
Bicyclo[2.2.1]heptan-2-amine, N,2,3,3-tetramethyl-, hydrochloride (1:1) (826-39-1) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RIDADR ]
UN 2811 6.1/PG 3
| [WGK Germany ]
3
| [RTECS ]
RB6900000
| [HazardClass ]
6.1 | [HS Code ]
2921300000 |
| Hazard Information | Back Directory | [Description]
Mecamylamine hydrochloride is a non-competitive nicotinic acetylcholine receptor antagonist with preferential activity at the α3β4 subtype (IC50 = 90-640 nM) compared to α4β2, α3β2, and α7 subtypes (IC50 range from 1-7 μM), previously used to treat hypertension.Displays antidepressant-like effects in mice.
Mecamylamine HCl is supplied as tablets for oral use, each containing 2.5 mg mecamylamine HCl. Inactive ingredients are calcium phosphate, D&C Yellow 10, FD&C Yellow 6, lactose, magnesium stearate, cornstarch, and talc. | [Chemical Properties]
Mecamylamine hydrochloride is a white, odorless, or practically odorless, crystalline powder, is highly stable, soluble in water and has a molecular weight of 203.75. | [Originator]
Inversine ,MSD ,US ,1956 | [Uses]
A noncompetitive nicotinic AChR inhibitor. | [Uses]
Mecamylamine hydrochloride has been used as an additive in extracellular saline during current-clamp recordings to reduce synaptic input. It has also been used as a non-selective nicotinic acetyl choline receptor blocker in aortic body neurons and in MLO-Y4 cells. | [Uses]
Mecamylamine is a noncompetitive nicotinic acetylcholine receptor antagonist with preferential activity at the α3β4 subtype (IC50 = 90-640 nM) compared to α4β2, α3β2, and α7 subtypes (IC50s range from 1-7 μM). Mecamylamine is widely used as a broad-spectrum antagonist of neuronal nicotinic acetylcholine receptors in basic nicotine research. It has been reported to be effective as an aid to smoking cessation and may also be of use in various nicotine-responsive, neuropsychiatric disorders.[Cayman Chemical] | [Definition]
ChEBI:Mecamylamine hydrochloride is a monoterpenoid. | [Manufacturing Process]
Preparation of 2-(N-Formylamino)Isocamphane: Into a 5-liter 3-necked round bottom flask equipped with stirrer, dropping funnel and thermometer, was added 325 ml of glacial acetic acid. Then, portionwise, a total of 133 g of sodium cyanide (granular, 2.6 mols) was added with stirring while holding the temperature at 15°C. To the thick white slurry was added dropwise a previously prepared cold mixture of 325 ml glacial acetic acid and 360 ml concentrated sulfuric acid.
After addition of a few milliliters at 15°C, the thick slurry thins slowly and the
remainder of the sulfuric-glacial acetic acid mixture was added at 0° to 2°C. A
total of about 2 hours was required for the addition. After addition, stirring
was continued for 15 minutes, Then dropwise, over an hour, a solution of 178
g (1.3 mold of dl-camphene in 50 ml of glacial acetic acid was added while
keeping the temperature at about 0°C (±3°C).
Stirring was continued for two hours at 0°C during which time a slight
pinkish-yellow color developed in the reaction mixture. The cooling bath was removed and the temperature allowed to rise to 15° to 20°C in about 2 to 3
hours. The ice bath was then re placed and while holding the temperature at
about 20°C, the mixture was gradually diluted with 3 liters of water while
stirring vigorously. After an hour or two of good agitation at room
temperature, the oily product was extracted with 2 x 500 ml and 1 x 200 ml
of chloroform and the combined extracts washed with 2 x 500 ml of water.
The chloroform extract was then rendered neutral by stirring with 500 ml
water and gradually adding solid sodium bicarbonate to the mixture until the
aqueous phase had a pH of about 7; required, approximately 88 g of NaHCO3.
After separation the chloroform layer was washed with 2 x 500 ml water, dried
over calcium chloride, and after filtration the solvent was removed in vacuo on
the steam bath. A solid somewhat sticky residue of 231.2 g was obtained.
After removal of last traces of chloroform by repeated swishing with petroleum
ether, the cake was finally refluxed with about 500 ml petroleum ether (BP
30° to 60°C) until a thick crystalline slurry was obtained. After refrigeration
for a day, the white crystalline mass was filtered by suction, washed with
petroleum ether (2 x 125 ml), then n-heptane (2 x 125 ml) and again with
petroleum ether (2 x 125 ml). After air drying at room temperature to
constant weight, 180.6 g of the dl-2-(N-formylamino)isocamphane melting at
160° to 165°C was obtained.
The combined petroleum ether and n-heptane washes were concentrated
under diminished pressure and the residual oil dissolved in a minimum
amount of hot petroleum ether (about 75 ml). The resulting solution was
placed in the refrigerator for two days. The precipitated dl-2-(N-formylamino)
isocamphane was then recovered by filtration and washed with petroleum
ether and n-heptane as described above. Obtained, 12.6 g of product having a
MP of 158° to 164°C.
The dl-2-(N-formylamino)isocamphane (193 g) was dissolved in 1.9 liters nheptane by heating on a steam bath, After clarifying the solution by filtration,
the clear filtrate was allowed to stand at room temperature until crystallization
was complete. The crystalline product is filtered by suction, washed with a
little cold n-heptane and air dried. The dl-2-(N-formylamino)isocamphane
melted at 169° to 174°C.
Preparation of 2-(N-Methylamino)Isocamphane: To 4.23 liters of anhydrous
ether in a 12-liter 3-necked flask fitted with a stirrer, reflux condenser and
dropping funnel was quickly added 78 g (2.05 mols) of lithium aluminum
hydride. The mixture was gently refluxed with stirring until all hydride had
dissolved which required several hours.
A solution of 168 g (0.92 mol) of dl-2-(N-formylamino)isocamphane, prepared
as described above, in 1.81 liters of anhydrous ether was then added during a
period of about one hour with stirring. After addition, the mixture was
refluxed for about 6 hours after which it was cooled slightly and 347 ml of
water added with stirring, hydrogen gas being evolved during the addition,
Stirring was continued until the precipitate changed to a powder, which was
filtered by suction and washed with ether (a total of about 2 liters).
The combined filtrate and washes were concentrated to 1.6 liters and the
concentrate containing the dl-2-(N-methylamino)isocamphane washed once
with about 350 cc water, and then dried over anhydrous sodium sulfate. The
dried ether concentrate was then cooled in an ice bath and with stirring a cold
saturated ethereal-hydrogen chloride solution was added slowly until acid to
Congo red; required, about 440 ml anhydrous ether saturated (at 0°C) with
HCl gas, After precipitation was complete, the white crystalline dl-2-(Nmethylamino)isocamphane hydrochloride was filtered, and washed with
anhydrous ether (about 1 liter) until the washes were neutral. The dl-2-(Nmethylamino)isocamphane hydrochloride was air dried at room temperature.
Obtained, 156.5 g of product melting with decomposition at 249°C. | [Therapeutic Function]
Antihypertensive | [General Description]
The secondary aminemecamylamine hydrochloride, N,2,3,3-tetramethyl-2-norbornanaminehydrochloride (Inversine), has a powerful ganglionicblocking effect that is almost identical to that ofhexamethonium. It has an advantage over most of the ganglionicblocking agents in being absorbed readily andsmoothly from the GI tract. It is rarely used, however, forthe treatment of moderate-to-severe hypertension becausesevere orthostatic hypotension occurs when the drug blockssympathetic ganglia. | [Biological Activity]
Non-competitive nicotinic acetylcholine receptor antagonist. Displays antidepressant-like effects in mice. | [Biochem/physiol Actions]
Noncompetitive nicotinic acetylcholine receptor antagonist; preferentially blocks nicotinic receptors at autonomic ganglia; crosses blood-brain barrier. | [Synthesis]
Mecamylamine, N,2,3,3-tetramethylnorbornan-2-ylamine (14.2.2), is
synthesized from 2,3,3-trimethylnorbornen-2, which is reacted in a Ritter reaction condi�tions with hydrogen cyanide in concentrated sulfuric acid, giving 2,3,3-trimethylnorbor�nan-2-ylformylamine (14.2.1), the reduction of which by lithium aluminum hydride leads
to mecamylamine (14.2.2) [32,33].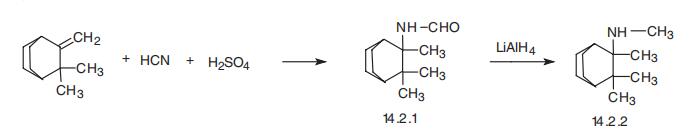
| [Metabolism]
Mecamylamine hydrochloride (Inversine) is a secondary
amine and can therefore easily penetrate cell membranes.
Its absorption from the gastrointestinal tract is more complete
than that of the quaternary ammonium compounds.
Mecamylamine is well absorbed orally and crosses both
the blood-brain and placental barriers; its distribution is
not confined to the extracellular space. High concentrations
of the drug accumulate in the liver and kidney, and
it is excreted unchanged by the kidney. In contrast to most
of the highly ionized ganglionic blocking agents, mecamylamine
can produce central nervous system effects, including
tremors, mental confusion, seizures, mania, and
depression.The mechanism by which these central effects
are produced is unclear.Mecamylamine is rarely used today
as an antihypertensive drug because it blocks both
parasympathetic and sympathetic ganglia. | [storage]
Store at 2-8°C |
|
|