| Identification | More | [Name]
Polychloroprene | [CAS]
9010-98-4 | [Synonyms]
CHLOROPRENE RESIN
NEOPRENE GNA
NEOPRENE GRT
NEOPRENE GW
NEOPRENE(R)
NEOPRENE TRT
NEOPRENE TW
NEOPRENE W
NEOPRENE WHV
NEOPRENE WRT
POLY(2-CHLORO-1,3-BUTADIENE)
POLYCHLOROPRENE
1,3-Butadiene,2-chloro-,homopolymer
1,4-cispoly(chloropene)
2-chloro-1,3-butadienehomopolymer
2-chloro-1,3-butadienepolymer
3-butadiene,2-chloro-homopolymer
3-butadiene,2-chloro-polymers
bostik1410
chlorobutadienepolymer | [EINECS(EC#)]
204-818-0 | [Molecular Formula]
C4H5Cl | [MDL Number]
MFCD00084406 | [MOL File]
9010-98-4.mol | [Molecular Weight]
88.5355 |
| Chemical Properties | Back Directory | [Appearance]
white to beige chips or chunks | [Melting point ]
>260 °C | [density ]
1.23 g/mL at 25 °C(lit.)
| [storage temp. ]
-196°C | [form ]
chunks
| [color ]
White to beige | [Dielectric constant]
6.0(Ambient) | [InChI]
InChI=1S/C4H5Cl/c1-3-4(2)5/h3H,1-2H2 | [InChIKey]
YACLQRRMGMJLJV-UHFFFAOYSA-N | [SMILES]
C=C(Cl)C=C | [Uses]
(Solid) Mechanical rubber products, lining
oil-loading hose and reaction equipment, adhesive
cement, binder for rocket fuels, coatings for electric
wiring, gaskets and seals. (Liquid) Specialty items
made by dipping or electrophoresis from the latex.
(Foam) Adhesive tape to replace metal fasteners for
automotive accessories, seat cushions, carpet backing, sealant | [CAS DataBase Reference]
9010-98-4(CAS DataBase Reference) | [IARC]
3 (Vol. 19, Sup 7) 1987 | [EPA Substance Registry System]
9010-98-4(EPA Substance) |
| Hazard Information | Back Directory | [Chemical Properties]
white to beige chips or chunks | [Definition]
ChEBI: A macromolecule composed of repeating (2Z)-2-chlorobut-2-ene-1,4-diyl units. | [Hazard]
Questionable carcinogen. | [Description]
Neoprene, also known as polychloroprene and chloroprene rubber, is an elastomer produced by polymerization of chloroprene. | [History]
Polychloroprene was discovered in 1930 at E. I. DuPont de Nemours & Co. inWilmington Delaware. The discovery grew out of a need to develop a synthetic substitute for natural rubber. DuPont first marketed this first commercially successful synthetic elastomer as DuPrene in 1933. In response to new technology development that significantly improved the product and manufacturing process, the name was changed to Neoprene in 1936. The current commercially acceptable generic name for this class of chlorinated elastomers is CR or chloroprene rubber.
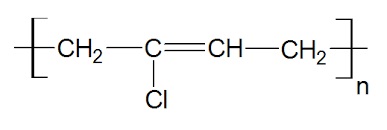
polychloroprene structure
| [Preparation]
Polychloroprene is made from one of two starting materials, acetylene or butadiene. Acetylene can be dimerized and then chloronated to form chloroprene. Alternatively, when adequate butadiene is available, this can be directly halogenated (eqs. 7 and 8). In either case, the chloroprene product can then be polymerized to polychloroprene. Essentially a butadiene elastomer with chlorine present in the backbone,the polymer exhibits excellent tensile strength and low hysteresis, much like natural rubber. Tensile strength properties up to 28 MPa are possible with the proper reinforcing system (see FILLERS). The polarity imparted by the chlorine atom improves the oil and solvent resistance approaching those of nitrile polymers. The polymer can be protected with para-phenylenediamine antiozonants to give ozone resistance, and heat aging is also good. As a result, chloroprene elastomers are used in a wide variety of applications needing a balance of such properties. | [Production Methods]
Commercial polychloroprene rubber is manufactured by aqueous free-radical emulsion polymerization followed by isolation of the solid polymer by one of several processes: freeze roll isolation, drum drying , extruder isolation, precipitation and drying or spray drying. Isolation of powdered polychloroprene has been reviewed. Of the methods cited, freeze roll and drum drying isolation are commercially important.
The large-scale commercial manufacture of polychloroprene consists of eight or nine unit operations:
(1) Monomer solution makeup Water solution makeup
(2) Emulsification
(3) Polymerization
(4) Stripping of residual monomer
(5) Peptization for chloroprene–sulfur copolymers
(6) Freeze roll isolation Drum drying
(7) Drying of freeze-rolled film
(8) Roping
(9) Cutting and packaging (25kg) | [Industrial uses]
One of the first synthetic rubbers used commercially to the rubber industry, neoprene is a polymer of chloroprene, 2-chlorobutadiene- 1,3. In the manufacturing process, acetylene, the basic raw material, is dimerized to vinylacetylene and then hydrochlorinated to the chloroprene monomer.
Sulfur is used to vulcanize some types of neoprene, but most of the neoprenes are vulcanized by the addition of basic oxides such as magnesium oxide and zinc oxide. The cure proceeds through reaction of the metal oxide with the tertiary allylic chlorine that arises from the small amount of 1,2-polymerization that occurs. Other compounding and processing techniques follow similar procedures and use the same equipment as for natural rubber. One of the outstanding characteristics of neoprene is the good tensile strength without the addition of carbon black filler. This versatility makes them useful in many applications requiring oil, weather, abrasion, or electrical resistance or combinations of these properties, such as wire and cable, hose, belts, molded and extruded goods, soles and heels, and adhesives. | [Materials Uses]
The most widely used contact adhesive is a solution of polychloroprene or modified polychloroprene in solvent blends of aromatic hydrocarbons, aliphatic hydrocarbons, esters, or ketones, for example, toluene–hexane–acetone. Viscosity, dry time needed before bonding, bond strength, and price are affected by the solvent. Using various combinations of the isomeric forms of polymerized 2- chlorobutadiene permits a fine-tuning of the crystallization rate of the dissolved polymer as the solvent evaporates. The polychloroprene may also be modified by the incorporation of methacrylic acid or mercaptans. Metal oxides (MgO and ZnO) that scavenge acids are often part of polychloroprene adhesives and also may act as cross-linking agents. Oxygen scavengers such as butylated hydroxytoluene (BHT) [128-37-0] or naphthylamines [25168-10-9] are added to prevent dehydrochlorination. To build initial handling strength, the solvent-based polychloroprene contact adhesives may be modified with alkyl phenolics, terpene phenolics, or phenolic-modified rosin esters, the first of these being the most effective and least deleterious. Chlorinated rubbers are sometimes added to these adhesives to improve their adhesion to plasticized PVC and other plastics. Added just before adhesive application, isocyanates are useful in modification of polychloroprene contact adhesives, reacting perhaps through hydrolysis of the pendant allylic groups present from the small number of 1,2 isomeric segments. The remainder of the solvent-based contact adhesives are comprised of polyurethane, SBR, styrene–butadiene–styrene block polymers, butadiene–acrylonitrile rubber, natural rubber, or various acrylic or vinyl resins in suitable solvents. | [Advantages]
By proper raw material selection and formulation of these polymers, the compounder can achieve optimum performance for a given end-use. Initially developed for resistance to oils and solvents it may resist various organic chemicals including mineral oils, gasoline, and some aromatic or halogenated solvents. It also exhibits good chemical resistance to aging and attack by ozone, and good resistance to UV irradiation (e.g., exposure to sunlight), until moderately elevated temperatures. Moreover, it has outstanding resistance to damage caused by flexing and twisting, an elevated toughness, and it resists burning but its electrical properties are inferior to that of natural rubber. Therefore, neoprene (polychloroprene) is noted for a unique combination of properties which has led to its use in thousands of applications throughout industry. |
|
|