114077-82-6
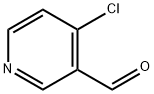 114077-82-6 结构式
114077-82-6 结构式
基本信息
4-氯吡啶-3-甲醛
4-氯-3-甲酰吡啶
4-氯-3-醛基吡啶
4-氯-3-吡啶甲醛
4-氯吡啶-3-甲醛 5G
4-氯-3-醛基吡啶/4-氯烟醛
4-CHLORO-3-FORMYLPYRIDINE
4-chloro-3-pyridylazaldehyde
4-chloropyridin-3-carbaldehyde
4-chloropyridine-3-carbaldehyde
4-chloro-3-pyridinecarbaldehyde
4-chloro-3-pyridincarboxaldehyde
4-Chloro-3-pyridinecarboxaldehyde
4-CHLOROPYRIDINE-3-CARBOXALDEHYDE
4-Chhloropyridine-3-carboxaldehyde
物理化学性质
制备方法
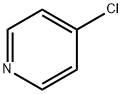
626-61-9
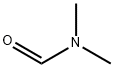
68-12-2

114077-82-6
一般步骤:在氩气保护下,将4-氯吡啶(25.0g,0.22mol)的四氢呋喃溶液(50ml)缓慢滴加至由1.6M正丁基锂的己烷溶液(179ml,0.29mol)与二异丙胺(33.4g,0.33mol)在四氢呋喃(300ml)中制备的二异丙基氨化锂溶液中,保持反应温度为-78℃。滴加完毕后,继续搅拌30分钟。随后,加入N,N-二甲基甲酰胺(DMF,19.3g,0.26mol),并将反应混合物逐渐升温至室温。反应完成后,用乙酸乙酯(200ml)和5%氯化铵水溶液(300ml)进行萃取。分离有机层,用无水硫酸镁干燥,减压浓缩除去溶剂,得到粗产物4-氯-3-吡啶甲醛(27g,收率86%),为油状物。产物经1H-NMR(200MHz,CDCl3)表征:δ 7.45(1H,d,J = 5.0Hz),8.69(1H,d,J = 5.0Hz),9.05(1H,s),10.51(1H,s)。
参考文献:
[1] Patent: EP1348706, 2003, A1. Location in patent: Page/Page column 37
[2] Patent: US2011/53975, 2011, A1. Location in patent: Page/Page column 60
[3] Journal of the American Chemical Society, 2013, vol. 135, # 40, p. 14916 - 14919
[4] Patent: WO2018/152329, 2018, A1. Location in patent: Page/Page column 44
[5] Patent: WO2004/41806, 2004, A2. Location in patent: Page 88-89
