180695-79-8
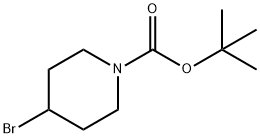 180695-79-8 结构式
180695-79-8 结构式
基本信息
N-BOC-4-溴哌啶
4-溴-N-BOC-哌啶
4-溴哌啶-1-甲酸叔丁酯
1-叔丁氧羰基-4-溴哌啶
4-溴-哌啶-1-羧酸叔丁酯
4-溴-N-BOC-哌啶1-BOC-4-溴哌啶
1-Boc-4-bromopiperidine
4-BroMo-N-Boc-piperidine
N-BOC-4-BROMO-PIPERIDINE
1-N-BOC-4-BROMOPIPERIDINE
1-tert-Butoxycarbonyl-4-bromopiperidine
tert-Butyl 4-bromopiperidine-1-carboxylate
1-Piperidinecarboxylic acid, 4-bromo-, 1,1-dimethylethyl ester
物理化学性质
制备方法

24424-99-5
54288-70-9

180695-79-8
步骤1:合成4-溴哌啶-1-甲酸叔丁酯 向含有5g(0.02mol)4-溴哌啶氢溴酸盐的35mL二氯甲烷悬浮液中,于0℃下缓慢滴加7.09mL(0.04mol)N,N-二异丙基乙胺。反应混合物搅拌30分钟后,继续滴加6.67g(0.031mol)二碳酸二叔丁酯的35mL二氯甲烷溶液。将反应混合物在室温下搅拌18小时。反应完成后,依次用1M盐酸水溶液(2×30mL)和盐水(30mL)洗涤反应混合物。分离有机层,用无水硫酸钠干燥,过滤后减压浓缩滤液,得到6.9g 4-溴哌啶-1-甲酸叔丁酯,为黄色油状物,产率定量。产物经1H NMR(250MHz,氯仿-d)表征:δ 1.46(9H,s),1.79-2.00(2H,m),2.00-2.16(2H,m),3.31(2H,ddd,J = 13.67,7.73,3.73 Hz),3.68(2H,ddd,J = 13.55,6.85,3.65 Hz),4.34(1H,tt,J = 7.69,3.81 Hz)。
参考文献:
[1] Patent: US2010/76029, 2010, A1. Location in patent: Page/Page column 42
[2] Patent: US2011/71196, 2011, A1. Location in patent: Page/Page column 22; 23
[3] European Journal of Medicinal Chemistry, 2018, vol. 158, p. 311 - 321
[4] Journal of the American Chemical Society, 2016, vol. 138, # 24, p. 7520 - 7523
[5] Organic Letters, 2000, vol. 2, # 1, p. 6 - 10

