30148-21-1
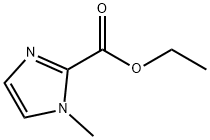 30148-21-1 结构式
30148-21-1 结构式
基本信息
1-甲基咪唑-2-甲酸乙酯
1-甲基咪唑-2-羧酸乙酯
乙基 1-甲基咪唑-2-羧酸酯
1-甲基-1H-咪唑-2-甲酸甲酯
1-甲基-1H-咪唑-2-甲酸乙酯
RARECHEM AL BI 0638
ETHYL 1-METHYLIMIDAZOLE-2-CARBOXYLATE
1--1H--2-MethyliMidazoleethyl forMate
1--1H- MethyliMidazole-2-ethyl forMate
2-(Ethoxycarbonyl)-1-methyl-1H-imidazole
ETHYL 1-METHYL-1H-IMIDAZOLE-2-CARBOXYLATE
Ethyl 1-methyl-1H-imidazole-2-carboxylate ,97%
1-Methyl-1H-iMidazole-2-carboxylic Acid Ethyl Ester
1H-Imidazole-2-carboxylic acid, 1-methyl-, ethyl ester
物理化学性质
制备方法
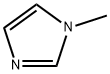
616-47-7
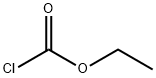
541-41-3

30148-21-1
1-甲基-1H-咪唑-2-羧酸乙酯的合成:在0℃条件下,将三乙胺(3.40 mL,24.4 mmol)和氯甲酸乙酯(2.34 mL,24.4 mmol)逐滴加入至1-甲基-1H-咪唑(1.00 g,12.2 mmol)的乙腈(4.0 mL)溶液中。反应混合物在室温下搅拌16小时。反应完成后,将混合物通过硅藻土过滤,滤液经减压浓缩除去溶剂。所得粗产物通过快速柱色谱法(硅胶为固定相,己烷/乙酸乙酯为洗脱剂)进行纯化,得到1-甲基-1H-咪唑-2-羧酸乙酯(1.50 g,9.73 mmol,产率80%)为白色固体。产物结构经1H-NMR(400 MHz,CDCl3)和ESI-MS确认:1H-NMR δ 1.42(3H,t,J = 7.2 Hz),4.01(3H,s),4.40(2H,q,J = 7.2 Hz),7.01-7.03(1H,m),7.13-7.15(1H,m);ESI-MS m/z = 155(M + H)+。
参考文献:
[1] Journal of the American Chemical Society, 1996, vol. 118, # 26, p. 6141 - 6146
[2] Patent: EP3263565, 2018, A1. Location in patent: Paragraph 0386; 0387
[3] Patent: JP2017/66077, 2017, A. Location in patent: Page/Page column 7; 8; 9; 10; 11
[4] Patent: WO2005/33077, 2005, A1. Location in patent: Page/Page column 53
[5] Patent: WO2004/58763, 2004, A1. Location in patent: Page 43
