800401-67-6
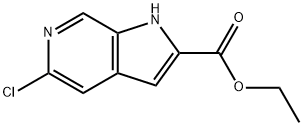 800401-67-6 结构式
800401-67-6 结构式
基本信息
5-氯-6-氮杂吲哚-2-羧酸乙酯
5-氯-1H-吡咯并[2,3-C]吡啶-2-甲酸乙酯
5-氯-1H-吡咯并[2,3-C]吡啶-2-甲酸乙酯:ETHYL 5-CHLORO-1H-PYRROLO[2,3-C]PYRIDINE-2-CARBOXYLATE
ethyl 5-chloro-1H-pyrrolo[2
5-Chloro-6-azaindole-2-carboxylic acid ethyl ester
ethyl 5-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxylate
5-chloro-1H-Pyrrolo[2,3-c]pyridine-2-carboxylic acid ethyl ester
1H-Pyrrolo[2,3-c]pyridine-2-carboxylic acid, 5-chloro-, ethyl ester
物理化学性质
制备方法
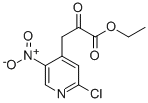
800401-66-5

800401-67-6
步骤2:合成5-氯-1H-吡咯并[2,3-c]吡啶-2-羧酸乙酯10-b 向3-(2-氯-5-硝基吡啶-4-基)-2-氧代丙酸乙酯10-a(2.726g,10mmol)的THF(80mL)和EtOH(30mL)溶液中加入饱和氯化铵溶液(50mL)。随后,在室温下于剧烈搅拌下分批加入铁粉(2.747g,4.9当量),然后将混合物加热回流2小时。反应完成后,将混合物冷却至室温,通过硅藻土过滤,并用温热的THF/乙醇(1:1,v/v)洗涤。蒸发滤液,将残余物溶于100mL水中,搅拌并回流。热过滤所得沉淀,用温水洗涤两次,然后真空干燥,得到5-氯-1H-吡咯并[2,3-c]吡啶-2-甲酸乙酯10-b(1.9g,收率84%)。 m/z = 225(M + H)+; 1H NMR(400MHz,DMSO-d6)δ ppm: 1.36(t,J = 6.8 Hz,3H),4.39(q,J = 6.7 Hz,2H),7.15(s,1H),7.76(s,1H),8.64(s,1H),12.59(br s,1H)。
参考文献:
[1] Patent: WO2012/80450, 2012, A1. Location in patent: Page/Page column 24
[2] Molecules, 2014, vol. 19, # 9, p. 13342 - 13357
[3] Patent: WO2006/59164, 2006, A2. Location in patent: Page/Page column 20
[4] Patent: WO2006/67532, 2006, A1. Location in patent: Page/Page column 19
[5] Patent: US2008/125459, 2008, A1. Location in patent: Page/Page column 9
