934524-10-4
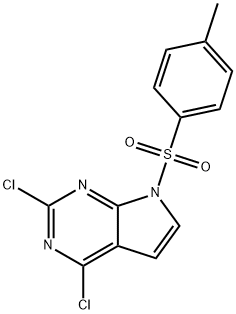 934524-10-4 结构式
934524-10-4 结构式
基本信息
2,4-二氯-7-对甲苯磺酸-7H-吡咯并[2,3-D]嘧啶
2,4-二氯-7-对甲基苯磺酰基-7H-吡咯[2,3-D]嘧啶
2,4-二氯-7-对甲苯磺酰基-7H-吡咯并[2,3-D]嘧啶
2,4-二氯-7-(甲苯-4-磺酰)-7H-吡咯并[2,3-D]嘧啶
2,4-Dichloro-7-tosyl-7H-pyrrolo[2,3-d]pyriMidine
2,4-Dichloro-7-(p-tolylsulfonyl)pyrrolo[2,3-d]pyrimidine
2,4-dichloro-7-(4-methylphenyl)sulfonylpyrrolo[2,3-d]pyrimidine
2,4-Dichloro-7-(toluene-4-sulfonyl)-7H-pyrrolo[2,3-d]pyriMidine
2,4-dichloro-7-(4-methylbenzenesulfonyl)-7H-pyrrolo[2,3-d]pyrimidine
2,4-Dichloro-7-[(4-methylphenyl)sulfonyl]-7H-pyrrolo[2,3-d]pyrimidine
7H-Pyrrolo[2,3-d]pyriMidine, 2,4-dichloro-7-[(4-Methylphenyl)sulfonyl]-
物理化学性质
制备方法
![2,4-二氯-7H吡咯[2,3-D]嘧啶](/CAS/GIF/90213-66-4.gif)
90213-66-4

98-59-9
![2,4-二氯-7-对甲苯磺酰基-7H-吡咯并[2,3-D]嘧啶](/CAS/GIF/934524-10-4.gif)
934524-10-4
以2,4-二氯-7H-吡咯并[2,3-d]嘧啶(1.00 g,5.32 mmol)和对甲苯磺酰氯(1.115 g,5.85 mmol)为原料,在室温下,将两者与硫酸氢四丁基铵(0.090 g,0.27 mmol)一同溶解于二氯甲烷(20 mL)中。随后,向反应混合物中加入50%氢氧化钠水溶液(1 mL),并在室温下搅拌30分钟。通过薄层色谱(TLC)监测反应进度,确认反应完成后,用去离子水和二氯甲烷稀释反应混合物,分离有机层。有机层经真空蒸发浓缩,得到浅黄色固体。该粗产物通过柱色谱法(100%二氯甲烷作为洗脱剂)纯化,得到目标产物2,4-二氯-7-甲苯磺酰-7H-吡咯并[2,3-d]嘧啶(1.76 g,收率97%),为白色固体。质谱(LCMS)显示:[M + H]+ m/z 342。1H NMR(400 MHz,氯仿-d)δ ppm:8.14(d,J = 8.59 Hz,2H),7.78(d,J = 3.79 Hz,1H),7.39(d,J = 8.59 Hz,2H),6.70(d,J = 3.79 Hz,1H),2.45(s,3H)。
参考文献:
[1] Patent: WO2010/38060, 2010, A1. Location in patent: Page/Page column 68-69
[2] Journal of Medicinal Chemistry, 2014, vol. 57, # 1, p. 144 - 158
[3] Patent: WO2010/123919, 2010, A2. Location in patent: Page/Page column 55
[4] Patent: WO2017/106771, 2017, A1. Location in patent: Paragraph 00397
[5] Patent: WO2017/133667, 2017, A1. Location in patent: Page/Page column 400