Trametinib Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Trametinib is an inhibitor of MEK1 and -2. It inhibits B-RAF- and C-RAF-induced phosphorylation of MEK1 (IC
50s = 3.4 and 1.8 nM, respectively) and MEK2 (IC
50s = 1.6 and 0.92 nM, respectively). Trametinib inhibits the growth of two human colorectal cancer cell lines expressing mutant B-RAF (IC
50s = 0.48 and 0.52 nM) and seven cell lines expressing mutant K-Ras (IC
50s = 2.2-174 nM) but does not inhibit the growth of wild-type COLO 320DM cells expressing both B-RAF and K-Ras (IC
50 = >10,000 nM). It reduces tumor growth in HT-29 and COLO 205 mouse xenograft models when used at doses of 0.3 and 1 mg/kg per day. Trametinib (0.03 and 0.1 mg/kg per day) also decreases
M. tuberculosis-induced increases in hind paw volume in a rat model of arthritis. Formulations containing trametinib, in combination with dabrafenib, have been used in the treatment of metastatic mutant B-RAF
V600E melanoma.
Verwenden
Used in the systematic treatment of advanced cutaneous melanoma. An EGFR kinase inhibitor.
Indications
MEK, also known as MAPK, is a dual specificity threonine/tyrosine kinase that is a key node in the Raf–Ras–MEK signaling pathway. Small-molecule MEK inhibitors represent the largest group of type III allosteric inhibitors that do not bind to the ATP binding pocket. As of December 2016, besides the FDA-approved MEK1/2 inhibitors trametinib (Mekinist(R), GlaxoSmithKline) and cobimetinib (Cotellic(R), Roche), over 10 MEK inhibitors are currently in clinical trials. Trametinib was approved by FDA in 2013 for the treatment of patients with either B-Raf V600E or V600K mutated metastatic melanoma. Considering the fact that MEK and Raf are different kinases along the same pathway of Ras–Raf–MEK/ERK signaling cascade, combination strategies using both MEK and B-Raf inhibitors were utilized to overcome the observed progression using single-agent trametinib, which usually occurs within 7months. FDA approved the combination of trametinib and dabrafenib for the treatment of B-Raf V600E/K mutated metastatic melanoma in January 2014 and the combination of cobimetinib and vemurafenib for the same type of indication. Although significant improvement in progression-free survival was observed using MEK/B-Raf combination strategy, the incidence of some common adverse effects, such as vomiting, diarrhea, nausea, rash, and pyrexia, also increased.
Definition
ChEBI: A pyridopyrimidine that is used (as its dimethyl sulfoxide addition compound) for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations, and who have not received prior BRAF inhibitor treatment.
Trademarks
Mekinist
Pharmakokinetik
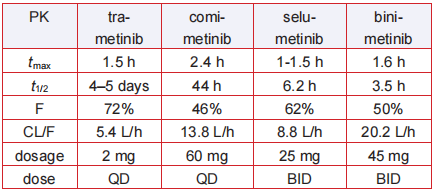
Trametinib has favorable pharmacokinetic
properties: quick oral absorption with tmax of 0.5–1.5
h, long duration of action with effective t1/2 of 4 days,
and elimination t1/2 of 10 days, as well as good oral
bioavailability (72%). The unusual long half-life and
good potency contribute a once-daily administration
of just 2 mg, the lowest dosage of all the MEK
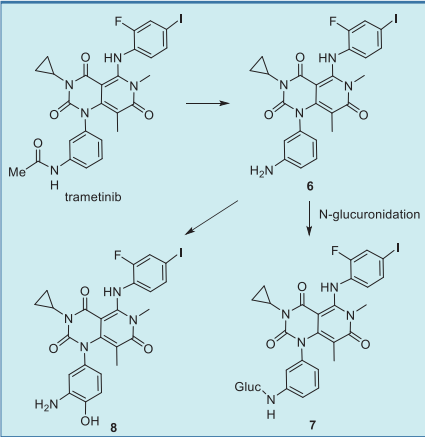
inhibitors now in use (Table 2). Trametinib is metabolized predominantly via
deacetylation to give 6, which subsequently
undergoes hydroxylation to give 8 or glucuronidation
to afford 7 (Fig. 4).
Clinical Use
Trametinib (GSK1120212) is an oral MEK inhibitor which has demonstrated
excellent results in combination therapy for BRAF-mutated melanoma and is FDA
approved in combination with BRAF inhibitors for that indication. Trametinib was
evaluated initially as a single agent in KRAS mutant NSCLC in comparison with
docetaxel and pemetrexed. Results as a single agent were not impressive, with an
ORR of only 12% in these patients.
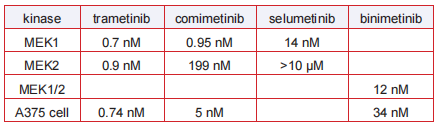
IC 50
In vitro IC50's of MEK inhibitors:
Trametinib Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

