Pixantrone Dimaleate Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Pixantrone dimaleate was approved by European Medicine Agency (EMA) on May 10, 2012. It was developed and marketed as Pixuvri® by Cell Therapeutics in EU. (CTI Life Sciences).
Pixantrone dimaleate is a DNA intercalating agent indicated as monotherapy for the treatment of adult patients with multiply relapsed or refractory aggressive Non-Hodgkin B-cell Lymphomas (NHL).
Pixuvri® is available as lyophilised powder for infusion, containing 29 mg of free Pixantrone. The recommended dose is 50 mg/m2 of pixantrone on days 1, 8 and 15 of each 28-day cycle for up to 6 cycles.
Chemische Eigenschaften
Blue Solid
Occurrence
Pixantrone dimaleate, also known as BBR 2778, was first synthesized by Professors Krapcho and Hacker at the University of Vermont. The University of Vermont and the Boehringer Mannheim Italia research center co-identified the in vitro tumor cell cytotoxicity.
Verwenden
Pixantrone is an antineoplastic drug belonging to group of antitumor antibiotics. Pixantrone is an anlogue of Mitoxantrone (M373425) and is just as potent in the treatment of multiple sclerosis with f
ewer toxic effects on cardiac tissue. Studies suggest that Pixantrone significantly reduces amyloid beta (A beta(1-42)) neurotoxicity, a mechanism implicated in Alzheimer's disease.
synthetische
The manufacturing scale synthesis of pixantrone dimaleate relies on several process modifications from the original synthesis reported by Krapcho in 1994. This modified procedure has provided active pharmaceutical ingredient (API) in high purity (>99%) and is acceptable for use in pharmaceutical applications (Scheme 25). Beginning with pyridine 3,4-dicarboxylic acid (129), generation of the corresponding anhydride 130 proceeded in 76% yield upon treatment with refluxing Ac2O. Next, an AlCl3-promoted Friedel–Crafts reaction of 1,4-difluorobenzene (131) with 130 under reflux conditions provided a mixture of nicotinic acid isomers 132a/132b in 84% yield, which were carried directly to the next step. Cyclization with fuming H2SO4 yielded the desired difluorobenzo-isoquinoline-dione core 133, which was further functionalized with ethylenediamine (134) to provide the free base of pixantrone. Subjection of the pixantrone free base to aqueous acetic anhydride and maleic acid provided pixantrone dimaleate (XX) in 92% yield over 3 steps.
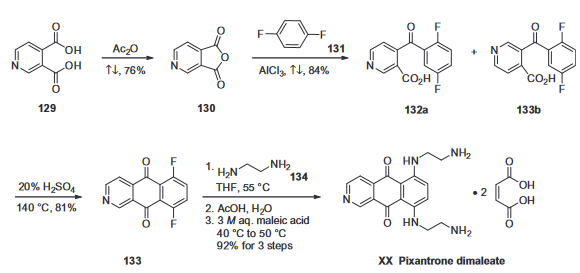
Synthesis of pixantrone dimaleate
Biologische Aktivität
Pixantrone (BBR 2778) is an aza-anthracenedione with enhanced antitumor activity due to its DNA-intercalating and topoisomerase II-poisoning activity. Pixantrone shows no signs of acute or delayed cardiotoxicity seen with anthracyclines mitoxantrone and doxorubicin (DOX), while exhibiting comparable in vivo efficacy against solid tumors in mice. Tests conducted on human myocardial strips ex vivo shows that not only pixantrone does not form superoxide anion and hydrogen peroxide (O2?? and H2O2) seen with DOX due to redox activation, pixantrone and its metabolites, especially N-dealkylated, show competitive inhibition against DOX reduction. While mitoxantrone does not form O2?? and H2O2 on its own, it synergizes with DOX to form more O2?? and H2O2, whose formation and subsequent production of the long-lived metabolite doxorubicinol contribute to DOX cardiotoxicity.
Einzelnachweise
[1] A. PAUL KRAPCHO. 6,9-Bis[(aminoalkyl)amino]benzo[g]isoquinoline-5,10-diones. A Novel Class of Chromophore-Modified Antitumor Anthracene-9,10-diones: Synthesis and Antitumor Evaluations[J]. Journal of Medicinal Chemistry, 1994, 37 6: 828-837. DOI:
10.1021/jm00032a018.
[2] 170. Krapcho, A. P.; Hacker, M. P.; Cavalletti, E.; Giuliani, F. C. US Patent 5587382 A,1996.
[3] Spinelli, S.; Didomenico, R. WO Patent 9526189 A1, 1995.
[4] Krapcho, P. A. EP Patent 503537 A1, 1992
Pixantrone Dimaleate Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

