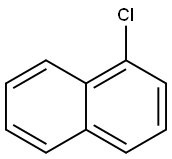
1-Chloronaphthalene
- CAS No.
- 90-13-1
- Chemical Name:
- 1-Chloronaphthalene
- Synonyms
- PCN-1;oronaphthaL;1-Chloronaph;1-Chlornaftalen;1-Chlornaphthalin;I-Chlornaphthalin;1-CHLORONAPHTHLENE;1-Naphtyl chloride;A-CHLORONAPHTALENE;1-chloronaphtalene
- CBNumber:
- CB1735332
- Molecular Formula:
- C10H7Cl
- Molecular Weight:
- 162.62
- MDL Number:
- MFCD00003874
- MOL File:
- 90-13-1.mol
- MSDS File:
- SDS
| Melting point | −20 °C(lit.) |
|---|---|
| Boiling point | 111-113 °C5 mm Hg(lit.) |
| Density | 1.194 g/mL at 25 °C(lit.) |
| vapor pressure | 0.05 hPa (20 °C) |
| refractive index |
n |
| Flash point | 250 °F |
| storage temp. | 2-8°C |
| solubility | alcohol: soluble |
| form | Liquid |
| color | Clear colorless to yellow, product may discolor during storage |
| PH | 7 (H2O, 20℃)(undiluted) |
| Water Solubility | insoluble. <0.1 g/100 mL at 20 ºC |
| Merck | 14,2149 |
| BRN | 970836 |
| Dielectric constant | 5.0 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 90-13-1(CAS DataBase Reference) |
| FDA UNII | K4OIF2EC56 |
| NIST Chemistry Reference | Naphthalene, 1-chloro-(90-13-1) |
| EPA Substance Registry System | 1-Chloronaphthalene (90-13-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335-H400 | |||||||||
| Precautionary statements | P273-P301+P312+P330-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,Xi,N,T,F | |||||||||
| Risk Statements | 22-36/37/38-51/53-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 26-36-61-37/39-29-36/37-45-16-7 | |||||||||
| RIDADR | 3082 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | QJ2100000 | |||||||||
| Autoignition Temperature | >500 °C | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29039990 | |||||||||
| Toxicity | LD50 orally in Rabbit: 1540 mg/kg LD50 dermal Rat > 2000 mg/kg | |||||||||
| NFPA 704 |
|
1-Chloronaphthalene price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.20315 | 1-Chloronaphthalene for synthesis | 90-13-1 | 100mL | $52.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.20315 | 1-Chloronaphthalene for synthesis | 90-13-1 | 1L | $154 | 2024-03-01 | Buy |
| Sigma-Aldrich | 185752 | 1-Chloronaphthalene technical grade | 90-13-1 | 100g | $45.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 185752 | 1-Chloronaphthalene technical grade | 90-13-1 | 500g | $151 | 2024-03-01 | Buy |
| TCI Chemical | C2310 | 1-Chloronaphthalene >97.0%(GC) | 90-13-1 | 5g | $87 | 2024-03-01 | Buy |
1-Chloronaphthalene Chemical Properties,Uses,Production
Description
The chlorinated naphthalenes in which one ormore hydrogen atoms have been replaced by chlorine toform wax-like substances, beginning with monochloronaphthalene and going on to the octachlor derivatives.Their physical states vary from mobile liquids to waxysolids depending on the degree of chlorination; Freezing/Melting points of the pure compounds range from 17℃for 1-chloronaphthalene to 198℃for 1,2,3,4-tetrachloronaphthalene.1-chloro-isomer: Hazard Identification (based on NFPA704 M Rating System): Health 2, Flammability 1,Reactivity 02-chloro-isomer: Molecular weight=162.62; Boilingpoint=256℃; Freezing/Melting point=61℃. HazardIdentification (based on NFPA 704 M Rating System):Health 2, Flammability 0, Reactivity 0. Insoluble in water.hexa-isomer: White to light-yellow solid with an aromaticodor. Molecular weight=334.82; Boiling point =343-388℃; Freezing/Melting point=137℃. Insoluble inwater.octa-isomer: Waxy, pale yellow solid with an aromaticodor. Molecular weight=403.74; Boilingpoint=410-440℃; Freezing/Melting point=185-192℃.Insoluble in water.penta-isomer: Colorless to white crystalline solid with abenzene-like odor. Molecular weight=300.40; Boilingpoint=336℃; Freezing/Melting point=120℃. Slightlysoluble in water.tetra-isomer: Colorless to pale yellow solid with an aromatic odor. Molecular weight=265.96; Boiling point =315-360℃; Freezing/Melting point=182℃; Flashpoint=210℃(oc). Insoluble in watertri-isomer: Colorless to pale yellow solid with an aromaticodor. Molecular weight=231.51; Boiling point =304-354℃; Freezing/Melting point=93℃; Flash point:198℃(oc). Insoluble in water.
Chemical Properties
The chlorinated naphthalenes in which one or more hydrogen atoms have been replaced by chlorine to form wax-like substances, beginning with monochloronaphthalene and going on to the octachlor derivatives. Their physical states vary from mobile liquids to waxysolids depending on the degree of chlorination. 1-Chloronaphthalene is a colorless volatile oily liquid. Insoluble in water, soluble in carbon tetrachloride, carbon disulfide, benzene and chlorobenzene.
Uses
1-Chloronaphthalene was widely used in Xylamits as a wood preservative with fungicidal and insecticidal properties in the past in Poland. 2-Chloronaphthalene was produced and used as a solvent in Poland, some of PCNs were found in polychlorinated biphenyls (PCBs) congeners.
Application
1-Chloronaphthalene was used as solvent additive in the fabrication of fullerene based photovoltaic devices. It was used in preparation of polyvinyl chloride based membranes of 5,10,15,20-tetrakis(4-methoxyphenyl)porphyrinatocobalt(II) for arsenite determination. Also used in the synthesis of pharmaceutical compounds such as aryl chlorides.
Synthesis Reference(s)
Journal of the American Chemical Society, 80, p. 1716, 1958 DOI: 10.1021/ja01540a052
The Journal of Organic Chemistry, 53, p. 2093, 1988 DOI: 10.1021/jo00244a046
Synthesis, p. 1155, 1985 DOI: 10.1055/s-1985-31461
General Description
1-chloronaphthalene is a clear colorless to amber oily viscous liquid. (NTP, 1992)
Air & Water Reactions
Insoluble in water.
Reactivity Profile
1-Chloronaphthalene is incompatible with strong oxidizing agents.
Fire Hazard
1-Chloronaphthalene is combustible.
Synthesis
1-Chloronaphthalene is obtained directly by chlorination of naphthalene, with the formation of more highly substituted derivatives such as dichloro- and trichloronaphthalenes in addition to the two monochlorinated isomeric compounds: 1-chloronaphthalene and 2-chloronaphthalene.
Reaction: Using zinc as catalyst, the ratio of naphthalene to chlorine is 1:0.84-1.17, the amount of catalyst is 0.4-0.5% of the weight of naphthalene, the chlorine passage time is 3-6h, the reaction temperature is 90-95℃, the average yield of 1-chloronaphthalene is 81.7%, After secondary fractionation, the product purity is 96%. The boiling point of 2-chloronaphthalene is similar to that of 1-chloronaphthalene, the chemical properties are similar, and it is difficult to separate.
Potential Exposure
Industrial exposure from individual chlorinated naphthalenes is rarely encountered; rather it usually occurs from mixtures of two or more Chlorinated naphthalenes. Due to their stability, thermoplasticity, and nonflammability, these compounds enjoy wide industrial application. These compounds are used in the production of electric condensers; in the insulation of electric cables and wires; as additives to extreme pressure lubricants; as supports for storage batteries; and as a coating in foundry use. octachloro-: Used as a fireproof and waterproof additive and lubricant additive. Pentachloro-: Used in electric wire insulation and in additives to special lubricants. tetrachloro-: Used in electrical insulating materials and as an additive in cutting oils. trichloro-: Used in lubricants and in the manufacture of insulation for electrical wire. Because of the possible potentiation of the toxicity of higher Chlorinated naphthalenes by ethanol and carbon tetrachloride, individuals who ingest enough alcohol to result in liver dysfunction would be a special group at risk. Individuals, e.g., analytical and synthetic chemists, mechanics and cleaners, who are routinely exposed to carbon tetrachloride or other hepatotoxic chemicals would also be at a greater risk than a population without such exposure. Individuals involved in the manufacture, utilization, or disposal of polychlorinated naphthalenes would be expected to have higher levels of exposure than the general population.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
storage
Color Code—Green: General storage may be used.Store in a refrigerator or a cool, dry place.
Shipping
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Wash the naphthalene with dilute NaHCO3, then dry it with Na2SO4 and fractionally distil it in vacuo. Alternatively, before distillation, it is passed through a column of activated alumina, or dried with CaCl2, then distilled from sodium. It can be further purified by fractional crystallisation by partial freezing or by crystallisation of its picrate to constant melting point (m 132-133o) from EtOH, and recovering it from the picrate. [Beilstein 5 H 541, 5 III 1570, 5 IV 1658.]
Degradation
As early as 1955, Walker and Wiltshire observed that 1-chloronaphthalene could be biodegraded. They obtained from soil samples two unidentified bacterial cultures which were able to use 1-chloronaphthalene as the sole source of carbon and energy. When grown on 1-chloronaphthalene the metabolites 8-chloro-1,2-dihydro-1,2-dihydroxynaphthalene and 3-chlorosalicylic acid were formed. The latter was proposed to be degraded to 3-chlorocatechol, which was further mineralized to carbon dioxide via the oltho-cleavage pathway. In another study, 7-chloro- 1,2-dihydro-1,2-dihydroxynaphthalene was produced during the bacterial degradation of 2-chloronaphthalene (Callahan et al . , 1979; Canonica et al., 1957).
Growth on both monochloronaphthalenes (each 1 mg/l) by a mixed bacterial culture was also observed by Okey and Bogan (1965). They found that 2-chloronaphthalene was metabolized faster than 1-chloronaphthalene. The results indicated that the mechanism of monochloronaphthalene degradation is similar to what is observed for naphthalene, and 1- and 2-methylnaphthalene (Mahajan et al., 1994).
Morris and Barnsley (1982) studied the cometabolic conversion of 2-chloronaphthalene in more detail. They obtained Pseudomonas strains which cometabolized both monochloronaphthalenes when grown on naphthalene and suggested that 2-monochloronaphthalene was metabolized to the intermediates 4-chlorosalicylic acid and 4-chlorocatechol. The latter intermediate was meta cleaved to 5-chloro-2-hydroxymuconic semialdehyde which was slowly metabolized further. This rate-limiting step probably prohibited growth on 2-chloronaphthalene.
Incompatibilities
All are incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Keep away from heat. Penta- is also incompatible with acids, alkalis.
Waste Disposal
High-temperature incineration with flue gas scrubbing. Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced.
1-Chloronaphthalene Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5873 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20293 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29798 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21667 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29888 | 58 |
| Xi'an Kono chem co., Ltd., | 029-86107037 13289246953 | info@konochemical.com | China | 2995 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49392 | 58 |
| Richest Group Ltd | 18017061086 | oled@richest-group.com | CHINA | 5601 | 58 |
View Lastest Price from 1-Chloronaphthalene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-08-15 | 1-Chloronaphthalene
90-13-1
|
US $10.00-1.00 / KG | 1KG | 0.99 | 30 tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-12-26 | 1-Chloronaphthalene
90-13-1
|
US $100.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-09-06 | 1-Chloronaphthalene
90-13-1
|
US $0.00-0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. |
-

- 1-Chloronaphthalene
90-13-1
- US $10.00-1.00 / KG
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- 1-Chloronaphthalene
90-13-1
- US $100.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- 1-Chloronaphthalene
90-13-1
- US $0.00-0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.