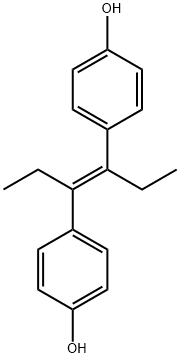
Diethylstilbestrol
- CAS No.
- 56-53-1
- Chemical Name:
- Diethylstilbestrol
- Synonyms
- DES;ALPHA;Estrogen;carbaldehyde;carboxaldehyde;piperidinamine;fluorobenzenamine;STILBESTROL;ylamine;Stilbetin
- CBNumber:
- CB9751457
- Molecular Formula:
- C18H20O2
- Molecular Weight:
- 268.35
- MDL Number:
- MFCD00002373
- MOL File:
- 56-53-1.mol
- MSDS File:
- SDS
| Melting point | 170-172 °C(lit.) |
|---|---|
| Boiling point | 351.51°C (rough estimate) |
| Density | 1.1096 (rough estimate) |
| refractive index | 1.4800 (estimate) |
| storage temp. | 2-8°C |
| solubility | methanol: 0.1 g/mL, clear, faintly yellow |
| form | Crystalline Powder |
| pka | pKa 9.02(H2O t=25 I=0.025) (Uncertain) |
| color | White to almost white |
| Water Solubility | PRACTICALLY INSOLUBLE |
| Merck | 13,3155 |
| BRN | 2056095 |
| Stability | Isomerizes rapidly in Benzene, Chloroform, and Ether. Keep Shielded from light. |
| CAS DataBase Reference | 56-53-1(CAS DataBase Reference) |
| FDA 21 CFR | 530.41 |
| EWG's Food Scores | 7-10 |
| NCI Dictionary of Cancer Terms | DES; diethylstilbestrol; estrogen |
| FDA UNII | 731DCA35BT |
| NCI Drug Dictionary | Acnestrol |
| ATC code | G03CB02,G03CC05,L02AA01 |
| Proposition 65 List | Diethylstilbestrol (DES) |
| NIST Chemistry Reference | Diethylstilbesterol(56-53-1) |
| IARC | 1 (Vol. 21, Sup 7, 100A) 2012 |
| EPA Substance Registry System | Diethylstilbestrol (56-53-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H319-H335-H350-H360FD-H410 | |||||||||
| Precautionary statements | P202-P261-P273-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 45-61-36/37/38-51/53-40 | |||||||||
| Safety Statements | 53-36/37/39-45-60-61-36/37 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | WJ5600000 | |||||||||
| F | 10 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29072990 | |||||||||
| Toxicity | LD50 oral in rat: > 3gm/kg | |||||||||
| NFPA 704 |
|
Diethylstilbestrol price More Price(63)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 46207 | Diethylstilbestrol VETRANAL , analytical standard | 56-53-1 | 250mg | $56.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1195001 | Diethylstilbestrol United States Pharmacopeia (USP) Reference Standard | 56-53-1 | 200mg | $381 | 2024-03-01 | Buy |
| Sigma-Aldrich | SAB1306249 | ANTI-DESMIN(C-TERMINAL) antibody produced in rabbit affinity isolated antibody, buffered aqueous solution | 56-53-1 | 400μL | $461 | 2023-01-07 | Buy |
| Sigma-Aldrich | SAB1306297 | ANTI-DESMIN (T16) antibody produced in rabbit affinity isolated antibody, buffered aqueous solution | 56-53-1 | 400μL | $427 | 2022-05-15 | Buy |
| Sigma-Aldrich | D4628 | Diethylstilbestrol ≥99% (HPLC) | 56-53-1 | 1g | $108 | 2024-03-01 | Buy |
Diethylstilbestrol Chemical Properties,Uses,Production
Description
Diethylstilbestrol (DES) is a synthetic estrogen receptor agonist that was prescribed to pregnant women in the late 1930s. It was banned in 1971 because of possible links to increased risk of breast cancer in mothers along with congenital abnormalities and increased risk of cancer in offspring. DES is structurally related to, and is as potent as, estradiol in most assays, with a longer half-
Chemical Properties
Diethylstilbestrol is an odorless, white crystalline powder, with a molecular weight of 268.36. Its cisisomer tends to revert to the trans-form. It is a nonsteroidal, synthetic stilbene derivative with estrogenic activity. recomended solvents are DMSO, DMF and ethanol, even in these solvents do not store in solution for any prolonged period of time.
Originator
DES,Amfre-Grant,US,1946
Uses
Diethylstilbestrol is a synthetic nonsteroidal estrogen that was formerly used in estrogenic hormone therapy (for menstrual disorders, postpartum breast engorgement, postcoital contraceptive, prevention of spontaneous abortion) and in chemotherapy of various cancers, including postmenopausal breast cancer and prostate cancer. It was also used in biomedical research and veterinary medicine (growth promoter for cattle and sheep; veterinary drug to treat estrogen deficiency disorders).
Uses
Diethylstilbestrol has been used:
to evaluate the estrogenic activity of diethylstilbestrol by quantitating the expression levels of endogenous estrogen-regulated marker genes
to evaluate the estrogenic, androgenic and toxicity responses in bioluminescent yeast bioreporter assay (BLYES)
to detect its effect on the proliferation and tyrosinase activity of melanocytes
Definition
ChEBI: Diethylstilbestrol is an olefinic compound that is trans-hex-3-ene in which the hydrogens at positions 3 and 4 have been replaced by p-hydroxyphenyl groups. It has a role as an antineoplastic agent, a carcinogenic agent, a xenoestrogen, an EC 3.6.3.10 (H(+)/K(+)-exchanging ATPase) inhibitor, an antifungal agent, an endocrine disruptor, an EC 1.1.1.146 (11beta-hydroxysteroid dehydrogenase) inhibitor, an autophagy inducer and a calcium channel blocker. It is a polyphenol and an olefinic compound.
Indications
Diethylstilbestrol is one of the older synthetic estrogens in use. It was used to treat prostate cancer but is now rarely used for this purpose because of its adverse effects,although it is occasionally used in postmenopausal women with breast cancer. It is taken orally in tablet form.
Manufacturing Process
50 parts by weight of p-hydroxypropiophenone are dissolved in 200 parts by weight of a 12.5% solution of caustic soda and shaken with 350 parts by
weight of 3% sodium amalgam. The sodium salt of the pinacol thereby
precipitating is reacted with glacial acetic acid, whereby the free pinacol is
obtained (MP 205°C to 210°C, after purification 215°C to 217°C). The yield
amounts to 95% of the theoretical. The pinacol is suspended in ether and
gaseous hydrogen chloride introduced, whereby water separates and the
pinacolin formed is dissolved in the ether, from which it is obtained by
evaporation as a viscous oil (diacetateof MP 91°C). The yield is quantitative.
40 parts by weight of pinacolin are dissolved in ethyl alcohol and gradually
treated with 80 parts by weight of sodium under reflux. The solution is
decomposed with water and the pinacolin alcohol formed extracted from the
neutralized solution with ether. The pinacolin alcohol is a viscous oil which is
characterized by a dibenzoate of MP 172°C. The yield is 95% of the
theoretical.
A solution of 30 parts by weight of pinacolin alcohol in ether is saturated with
hydrogen chloride at room temperature and the ether solution then agitated
with bicarbonate. After concentration by evaporation it leaves behind the
crude diethylstilbestrol [α,β-(p,p'-dihydroxydiphenyl)-α,β-diethylethylene]
which, when recrystallized from benzene, melts at 170°C to 171°C. The yield
amounts to 75% of the calculated. The total yield of diethylstilbestrol,
calculated on p-hydroxypropiophenone, is 68% of the theoretical.
brand name
Stilbestrol (Tablicaps); Stilbetin (Bristol-Myers Squibb);Distilbene;Oestro-gynedron;Stilphostrol.
Therapeutic Function
Estrogen
World Health Organization (WHO)
Diethylstilbestrol, a synthetic estrogen which is a stilbene derivative, was introduced into obstetric practice in the late 1940s and subsequently widely used for the treatment of threatened abortion. This use was later shown to be associated with an increased risk of vaginal cancer in the offspring which resulted in restrictive regulatory action in several countries. Diethylstilbestrol and other stilbenes remain available in many countries, however, for the treatment of certain hormone-dependent neoplasms including carcinoma of the prostate and postmenopausal breast cancer. (Reference: (WHODI) WHO Drug Information, 77.1, 16, 1977)
General Description
Diethylstilbestrol is an odorless tasteless white crystalline powder. (NTP, 1992)
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Diethylstilbestrol is incompatible with strong oxidizing agents, strong bases, acid chlorides and acid anhydrides .
Fire Hazard
Flash point data for Diethylstilbestrol are not available; however, Diethylstilbestrol is probably combustible.
Biochem/physiol Actions
Diethylstilbestrol is a synthetic estrogen with carcinogenic properties. Causes renal clear-cell carcinoma in Syrian hamster. In humans it causes increased risk of breast cancer, clear cell adenocarcinoma (CCA) of the vagina and cervix, and reproductive anomalies. Used in the treatment of prostate cancer to block the production of testosterone.
Side effects
These include sodium retention and oedema,nausea,gynaecomastia and impotence in men, and venous and arterial thrombosis. It can cause bone pain and hypercalcaemia when used for breast cancer.
Safety Profile
Confirmed carcinogen producing skin, liver, and lung tumors in exposed humans as well as uterine and other reproductive system tumors in the female offspring of exposed women. Experimental carcinogenic, neoplas tigenic, tumorigenic, and teratogenic data. A transplacental carcinogen. A human teratogen by many routes. Poison by intraperitoneal and subcutaneous routes. It causes glandular system effects by sktn contact. Human reproductive effects by ingestion: abnormalspermatogenesis; changes in testes, epididymis, and sperm duct; menstrual cycle changes or disorders; changes in female ferulity; unspecified maternal effects; developmental abnormalities of the fetal urogenital system; germ cell effects in offspring; and delayed effects in newborn. Implicated in male impotence and enlargement of male breasts. Other experimental reproductive effects. Mutation data reported. When heated to decomposition it emits acrid smoke and fumes. See also ETHINYL ESTRADIOL.
Potential Exposure
DES had been used extensively as agrowth promoter for cattle and sheep and is used in veterinary medicine to treat estrogen-deficiency disorders. It hasbeen used in humans to prevent spontaneous abortions; totreat symptoms associated with menopause, menstrual disorders, postpartum breast engorgement, primary ovarianfailure, breast cancer, and prostate cancers in males. In1979, the DFA revoked all use of DES in food-producinganimals. The Court of Appeals upheld the Commissioner’sdecision to revoke the use of DES in all food-producing animals on November 24, 1980; the motion to reconsider wasdenied on December 24, 1980.
First aid
Skin Contact: Flood areas of body that havecontacted the substance with water. Do not wait to removecontaminated clothing; do it under the water stream. Usesoap to help assure removal. Isolate contaminated clothingwhen removed to prevent contact by others. Eye Contact:Remove any contact lenses at once. Flush eyes well withcopious quantities of water or normal saline for at least20-30 min. Seek medical attention. Inhalation: Leave contaminated area immediately, breathe fresh air. Proper respiratory protection must be supplied to any rescuers. Ifcoughing, difficult breathing, or any other symptomsdevelop, seek medical attention at once even if symptomsdevelop many hours after exposure. Ingestion: If convulsions are not present, give a glass or two of water or milk todilute the substance. Assure that the person’s airway isunobstructed and contact a hospital or poison center immediately for advice on whether or not to induce vomiting.
Carcinogenicity
Diethylstilbestrol is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans.
Environmental Fate
Diethylstilbestrol is a known teratogen and carcinogen. Experimental studies using transgenic estrogen receptor knockout animals suggest that binding and activation of the estrogen receptor is required to elicit diethylstilbestrol toxicity. Hence, diethylstilbestrol lesions primarily appear in tissues enriched with estrogen receptors. Diethylstilbestrol binds to the estrogen receptor with a very high affinity and forms a complex with the target tissue. The complex then internalizes in to the cell and translocates to the nucleus. Once in the nucleus, diethylstilbestrol may directly bind with the cellular DNA and cause mutations and unscheduled DNA synthesis. Diethylstilbestrol is also known to induce aneuploidy.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Color Code—Green: Generalstorage may be used. Prior to working with DES you shouldbe trained on its proper handling and storage. Store in arefrigerator under an inert atmosphere and protect fromlight. A regulated, marked area should be established wherethis chemical is handled, used, or stored in compliance withOSHA Standard 1910.1045.
Shipping
The name of this material is not on the DOT listof materials for label and packaging standards. However,based on regulations, it may be classified as anEnvironmentally hazardous substances, solid, n.o.s. Thischemical requires a shipping label of “CLASS 9.” It falls inHazard Class 9 and Packing Group III.[20,21]
Toxicity evaluation
Diethylstilbestrol’s production and use in biochemical research, medicine, and veterinary medicine may result in its release to the environment through various waste streams. It may also be released to the environment during transport, storage, or disposal. If released to soil, diethylstilbestrol is predicted to strongly adsorb to the soil. Volatilization from the dry or wet soil surface would probably be unlikely. The extent of biodegradation in soil is not known, although diethylstilbestrol has been shown to be resistant to degradation in activated sludge. If released to water, diethylstilbestrol may bioconcentrate in aquatic organisms and strongly adsorb to suspended solids and sediments. Diethylstilbestrol is expected to be essentially nonvolatile on water surfaces. Diethylstilbestrol would not be susceptible to hydrolysis. The extent of biodegradation in natural waters is not certain, although diethylstilbestrol has been shown to be resistant to degradation in activated sludge. If released to the atmosphere, diethylstilbestrol vapors should rapidly oxidize, primarily by reaction with ozone. It is expected to exist solely in the particulate phase in an ambient atmosphere. Particulatephase diethylstilbestrol may be removed from the air by wet and dry deposition.
Clinical claims and research
At first glance, it might be surprising that synthetic nonsteroidal molecules such as diethylstilbestrol (DES) could have the same activity as estradiol or other estrogens. DES can be viewed, however, as a form of estradiol with rings B and C open and a six-carbon ring D. The activity of DES analogs was explained in 1946. It was proposed that the distance between the two DES phenol OH groups was the same as the 3-OH to 17-OH distance of estradiol; therefore, they could both fit the same receptor. Medicinal chemists have shown the OH-to-OH distance to be actually 12.1 ? in DES and 10.9 ? in estradiol. In aqueous solution, however, estradiol has two water molecules that are hydrogen bonded to the 17-OH. If one of the two water molecules is included in the distance measurement, there is a perfect fit with the two OH groups of DES. This suggests that water may have an important role for estradiol in its receptor site.
It is now generally accepted that the estrogens must have a phenolic moiety for binding, but some investigators propose that the receptor may be flexible enough to accommodate varying distances between the two key hydroxyls. This point about estrogens needing a phenolic ring for high-affinity binding to the ER is critical. Steroids with a phenolic A ring and related phenolic compounds lack high-affinity binding to the other steroid hormone receptors.
Diethylstilbestrol Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | sales@myuchem.com | China | 6710 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-13102810335 | admin@hbsaisier.cn | China | 747 | 58 |
| Hubei XinRunde Chemical Co., Ltd. | +8615102730682 | bruce@xrdchem.cn | CHINA | 566 | 55 |
| Nanjing Finetech Chemical Co., Ltd. | 025-85710122 17714198479 | sales@fine-chemtech.com | CHINA | 885 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Hebei shuoxi biotechnology co. LTD | +8613081092107 | CHINA | 968 | 58 |
Related articles
- Toxicity of Diethylstilbestrol
- Diethylstilbestrol (DES) is a nonsteroidal, synthetic stilbene derivative with estrogenic activity. It is an odorless, white c....
- Jan 19,2022
View Lastest Price from Diethylstilbestrol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-12 | Diethylstilbestrol
56-53-1
|
US $6.00 / KG | 1KG | More than 99% | 2000KG/Month | Hebei Saisier Technology Co., LTD | |
 |
2024-03-16 | Diethylstilbestrol | US $0.00 / KG | 100g | 98%+ | 100kg | WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD | |
 |
2024-02-23 | Diethylstilbestrol
56-53-1
|
US $83.00-376.00 / g | 25g | 0.99 | 25kg | Shanghai UCHEM Inc. |
-

- Diethylstilbestrol
56-53-1
- US $6.00 / KG
- More than 99%
- Hebei Saisier Technology Co., LTD
-

- Diethylstilbestrol
- US $0.00 / KG
- 98%+
- WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD
-

- Diethylstilbestrol
56-53-1
- US $83.00-376.00 / g
- 0.99
- Shanghai UCHEM Inc.
56-53-1(Diethylstilbestrol)Related Search:
1of4