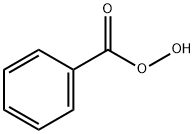
Perbenzoic acid
- CAS No.
- 93-59-4
- Chemical Name:
- Perbenzoic acid
- Synonyms
- peroxybenzoic acid;phenyl hydrogen carbonate;Benzoyl hydroperoxide;BzPo;Perbenzoic acid;Benzenecarboperoxoic acid;carbonic acid phenyl ester;XCRBXWCUXJNEFX-UHFFFAOYSA-N
- CBNumber:
- CB1852437
- Molecular Formula:
- C7H6O3
- Molecular Weight:
- 138.12
- MDL Number:
- MFCD00216871
- MOL File:
- 93-59-4.mol
| Melting point | 41-43° |
|---|---|
| Boiling point | bp15 100-110° (partial decomposition) |
| Density | 1.2667 (rough estimate) |
| refractive index | 1.4600 (estimate) |
| pka | 7.98±0.40(Predicted) |
| color | Leaflets or platelets from pet ether |
| CAS DataBase Reference | 93-59-4 |
| EWG's Food Scores | 1 |
| FDA UNII | C0EZ97V99I |
Perbenzoic acid Chemical Properties,Uses,Production
Chemical Properties
Volatile solid with a pungent odor; sublimes;mp 41–43°C (105.8–109°F); bp 100–105°C(212–221°F) at 15 torr (partially decomposes); slightly soluble in water but mixesreadily with most organic solvents.
Uses
Peroxybenzoic acid is used in organic analyses to measure the degree of unsaturation,and in making epoxides.
Uses
Perbenzoic acid is used to convert ethylenic compounds into oxides; in analysis of unsatd compounds, to determine the number of double bonds.
Synthesis Reference(s)
Tetrahedron, 23, p. 3327, 1967 DOI: 10.1016/S0040-4020(01)92300-2
Health Hazard
The toxicity of peroxybenzoic acid is verylow on animals. In humans it is almostnontoxic. Peroxybenzoic acid caused skintumor in mice on prolonged contact (NIOSH1986). Its tumorigenic action was lower thanthat of peroxyacetic acid. Its carcinogenicityin humans is unknown.
Fire Hazard
The presence of the peroxide functional group renders its strong oxidizing properties similar to those of other peroxy compounds. However, this compound is not hazardous like peroxyacetic or peroxyformic acids. It is not shock sensitive. Violent decomposition may occur only at high temperatures and/or in conjunction with certain organic contaminants that are easily oxidizable. Although there is no report of its explosion, general safety measures for handling peroxy compounds should be followed.
Safety Profile
Moderately irritating to skin, eyes, and mucous membranes by ingestion and inhalation. Questionable carcinogen with experimental tumorigenic data by skin contact. A dangerous fire hazard when exposed to heat, flame, or reducing materials. A powerful oxid
Purification Methods
Crystallise the peracid from *benzene or pet ether. It sublimes readily and is steam volatile. It is soluble in CHCl3, CCl4 and Et2O. [Braun Org Synth Coll Vol I 431 1941.] EXPLOSIVE.
Perbenzoic acid Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9020 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Shandong Xiya Chemical Co., Ltd | 13355009207 13355009207 | 3007715519@qq.com | China | 18739 | 57 |
| QUALITY CONTROL SOLUTIONS LTD. | 13670046396 | ORDERS@QCSRM.COM | China | 18820 | 58 |
| Supplier | Advantage |
|---|---|
| Shaanxi Didu New Materials Co. Ltd | 58 |
| Mainchem Co., Ltd. | 55 |
| Shandong Xiya Chemical Co., Ltd | 57 |
| QUALITY CONTROL SOLUTIONS LTD. | 58 |
93-59-4(Perbenzoic acid)Related Search:
1of4