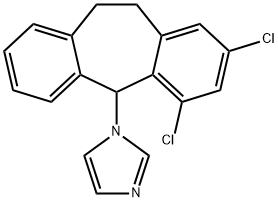
Eberconazole
- CAS No.
- 128326-82-9
- Chemical Name:
- Eberconazole
- Synonyms
- Eberconazole;1H-Imidazole, 1-(2,4-dichloro-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-yl)-;(+/-)-1-(2,4-Dichloro-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-yl)imidazole
- CBNumber:
- CB31178592
- Molecular Formula:
- C18H14Cl2N2
- Molecular Weight:
- 329.22
- MDL Number:
- MFCD00865628
- MOL File:
- 128326-82-9.mol
- MSDS File:
- SDS
| Boiling point | 495.0±45.0 °C(Predicted) |
|---|---|
| Density | 1.35±0.1 g/cm3(Predicted) |
| pka | 5.84±0.10(Predicted) |
| FDA UNII | V7O1U41C9B |
| ATC code | D01AC17 |
Eberconazole price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| American Custom Chemicals Corporation | HCH0122631 | (+/-)-1-(2,4-DICHLORO-10,11-DIHYDRO-5H-DIBENZO(A,D)CYCLOHEPTEN-5-YL)IMIDAZOLE 95.00% | 128326-82-9 | 5MG | $497.16 | 2021-12-16 | Buy |
| Product number | Packaging | Price | Buy |
|---|---|---|---|
| HCH0122631 | 5MG | $497.16 | Buy |
Eberconazole Chemical Properties,Uses,Production
Description
Eberconazole is a new member of the azole class of antifungal agents, and it is indicated for the topical treatment of cutaneous fungal infections, including tinea corporis (ringworm of the body), tinea cruris (ringworm of the groin) and tinea pedis (athlete’s foot) infections. Its mode of action is similar to that of other azole antifungals, namely inhibition of fungal lanosterol 14α-demethylase. Eberconazole exhibits good in vitro activity against a wide range of Candida species, including Candida. tropicalis, dermatophytes and Malassezia spp. yeasts. It shows good activity against Candida. Parapsilosis (MIC90=0.125 mg/mL), which is a relevant species in skin and nail disorders. In addition, eberconazole is effective against some of the highly triazole-resistant yeasts such as Candida. glabrata and Candida. krusei, as well as fluconazole-resistant Candida. albicans. However, eberconazole is less active than clotrimazole and ketoconazole against Candida. neoformans and a number of clinically relevant molds. Eberconazole is supplied as a 1% or 2% cream, and the topical application does not result in detectable serum, urine, or fecal levels. In a phase II study of 60 patients with tinea corporis and tinea cruris, treatment with topical eberconazole (1% or 2% cream), applied once or twice daily for 6 weeks, resulted in cure rates ranging from 73.3–93.3% at the end of therapy, and 66.7–100% six weeks post-therapy.
Originator
Chiesi Wassermann (Spain)
Uses
Eberconazole can be used for a novel antifungal formulation.
Definition
ChEBI: 1-(2,4-dichloro-10,11-dihydrodibenzo[a,d][7]annulen-5-yl)imidazole is a member of the class of dibenzannulenes that is 10,11-dihydrodibenzo[a,d][7]annulene carrying two chloro substituents at positions 2 and 4 as well as an imidazol-1-yl substituent at position 5. It is a member of imidazoles, an organochlorine compound and a dibenzannulene. It derives from a hydride of a dibenzo[a,d][7]annulene.
brand name
Ebernet
Synthesis
The synthesis of Eberconazole started with the Wittig reaction of the phosphonium bromide 43 with the 3,5-dichlorobenzaldehyde to give the olefin mixture 44. Hydrolysis of the ester followed by hydrogenation gives acid 46, which was cyclized to tricyclized ketone 47. Completion of the synthesis was accomplished in three steps via reduction of the ketone 47 with sodium borohydride, chlorination of resulting alcohol 48 with thionyl chloride and alkylation of the chloride 49 with imidazole to give eberconazole (VII).
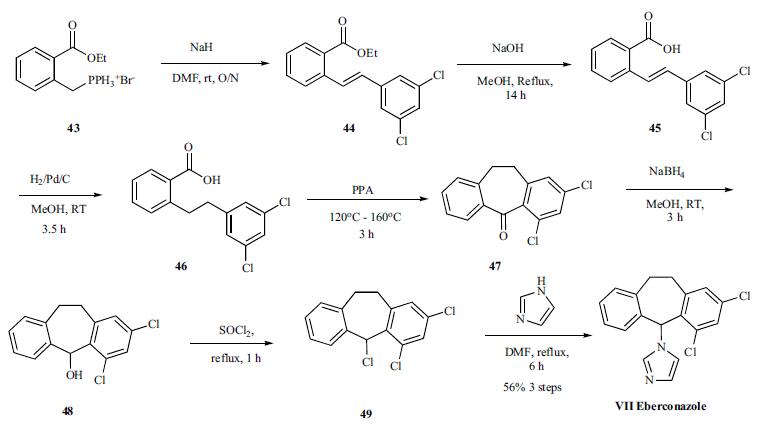
Eberconazole Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| QUALITY CONTROL SOLUTIONS LTD. | 0755-66853366 15920483169 | sales@chem-strong.com | China | 18810 | 58 |
| Hubei Qingbei Yunyan Pharmaceutical Technology Co., Ltd | 18162595016 | 3287908757@qq.com | China | 9514 | 58 |
| Wuhan Yuancheng Gongchuang Technology Co., Ltd. | 17683743113 | 1414717232@qq.com | China | 6167 | 58 |