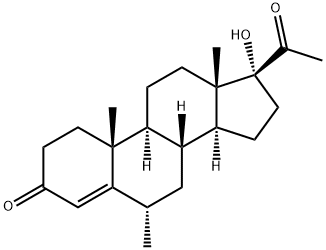
MEDROXYPROGESTERONE
- CAS No.
- 520-85-4
- Chemical Name:
- MEDROXYPROGESTERONE
- Synonyms
- (-)-Allantoin;u8840;farlutal;NSC 27408;Medoxyprogesterone;medroxyprogesteron;6-DIHYDROMEGESTROL;MEDROXYPROGESTERONE;METHYLOXYPROGESTERONE;Medroxyprogesteronebase
- CBNumber:
- CB3419654
- Molecular Formula:
- C22H32O3
- Molecular Weight:
- 344.49
- MDL Number:
- MFCD00069474
- MOL File:
- 520-85-4.mol
| Melting point | 204-206°C |
|---|---|
| alpha | D25 +75° (in chloroform) |
| Boiling point | 419.57°C (rough estimate) |
| Density | 1.0906 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 13.03±0.70(Predicted) |
| color | White |
| Merck | 13,5817 |
| BRN | 2510965 |
| CAS DataBase Reference | 520-85-4(CAS DataBase Reference) |
| FDA UNII | HSU1C9YRES |
| ATC code | G03AC06,G03DA02,L02AB02 |
| EPA Substance Registry System | Medroxyprogesterone (520-85-4) |
MEDROXYPROGESTERONE price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 1378023 | Medroxyprogesterone acetate Related Compound B United States Pharmacopeia (USP) Reference Standard | 520-85-4 | 25mg | $1300 | 2024-03-01 | Buy |
| Sigma-Aldrich | 46411 | Medroxyprogesterone VETRANAL | 520-85-4 | 100mg | $142 | 2022-05-15 | Buy |
| TCI Chemical | M3113 | Medroxyprogesterone >98.0%(HPLC) | 520-85-4 | 1g | $174 | 2024-03-01 | Buy |
| TCI Chemical | M3113 | Medroxyprogesterone >98.0%(HPLC) | 520-85-4 | 5g | $601 | 2024-03-01 | Buy |
| Cayman Chemical | 24908 | Medroxyprogesterone ≥98% | 520-85-4 | 100mg | $61 | 2024-03-01 | Buy |
MEDROXYPROGESTERONE Chemical Properties,Uses,Production
Chemical Properties
MEDROXYPROGESTERONE is White Solid
Originator
Provera,Upjohn,US,1959
Uses
An orally active progestogen used in hormone replacement therepy (HRT), in the past has been used as a component of oral contraceptives.SMedroxyprogesterone Acetate USP Related Compound B.
Uses
MEDROXYPROGESTERONE is an orally active progestogen used in hormone replacement therepy (HRT), in the past has been used as a component of oral contraceptives.
Definition
ChEBI: A 3-oxo Delta4-steroid that is pregn-4-ene-3,20-dione substituted by an alpha-hydroxy group at position 17 and a methyl group at position 6.
Manufacturing Process
Preparation of 17α-Hydroxyprogesterone 3,20-Bis-(Ethylene Ketal): A solution was prepared containing 50.0 g of 17α-hydroxyprogesterone in 1,000 ml of benzene, 100 ml of ethylene glycol and 2.5 g of p-toluenesulfonic acid monohydrate. This mixture was refluxed for a period of 17 hours using a calcium carbide water-trap to remove the water formed in the reaction. After this period of reflux 6.5 ml of pyridine was added to the solution, and the mixture cooled to room temperature.
The lower glycol layer was separated and washed with benzene. The benzene
layer and the benzene washings were combined and the combined solution
was divided into two equal portions, one of which was used for the isolation of
17α-hydroxyprogesterone 3,20-bis-(ethylene ketal) as follows. The benzene
solution was washed with 5% sodium carbonate solution, water and saturated sodium chloride solution. After being dried over anhydrous magnesium sulfate
the solution was concentrated to dryness at reduced pressure, The residue
was recrystallized by taking up in hot methylene chloride, adding acetone and
boiling to remove the methylene chloride until a final volume of about 200 ml
was reached.
The solution was then refrigerated overnight and 17.8 g of crystals were
removed by filtration. A second crop was obtained yielding 3.7 g of
compound. The total yield of 17α-hydroxyprogesterone 3,20-bis-(ethylene
ketal) was 20.3 g (64.3% of theory). Recrystallization of the crude 17αhydroxyprogesterone 3,20-bis-(ethylene ketal) from methanol gave the pure
bisketal of MP 209° to 211°C.
Preparation of 5α,6α-Oxido-17α-Hydroxyallopregnane-3,20-dione 3,20-Bis-
(Ethylene Ketal): A solution was prepared by heating 19.96 g (0.0477 mol) of
17α-hydroxyprogesterone 3,20-bis-(ethylene ketal) and 500 ml of benzene.
After the solution was effected the flask was cooled to 5°C and a mixture of
3.68 g (0.0449 mol) of sodium acetate and 174 ml of 40% peracetic acid was
added with stirring. The reaction mixture was stirred in the ice bath for 3
hours. The lower peracid layer was separated, diluted with water and
extracted twice with benzene.
The upper layer was neutralized by the addition of cold 10% sodium hydroxide
solution while stirring in an ice bath. The rate of addition of the sodium
hydroxide was regulated to keep the temperature below 10°C. The benzene
extracts from the peracid layer were combined and washed with cold 10%
sodium hydroxide solution and with saturated sodium chloride solution. All the
aqueous layers were washed again with the same portion of benzene. The
combined benzene layers were dried over anhydrous magnesium sulfate and
concentrated to dryness at reduced pressure.
The residue was recrystallized from acetone using methylene chloride to aid in
solution. The crystalline material was removed by filtration and was
recrystallized from methylene chloride-acetone to yield a total of 8 g of 5α,6αoxido-17α-hydroxyallopregnane-3,20-dione 3,20-bis-(ethylene ketal) of MP
211° to 215°C. For analytical purposes, another recrystallization from
methylene chloride-acetone gave pure 5α,6α-oxido-17α-hydroxyallopregnane3,20-dione 3,20-bis-(ethylene ketal) of MP 216° to 218.5°C.
Preparation of 5α,17α-Dihydroxy-6β-Methylallopregnane-3,20-dione 3,20-bis-
(Ethylene Ketal): To a solution of 91.6 g of 5α,6α-oxido-17αhydroxyallopregnane-3,20-dione 3,20-bis-(ethylene ketal) in 3,500 ml of
freshly distilled tetrahydrofuran was added 1,170 ml of commercial 3 molar
methyl magnesium bromide in ether solution. The reaction mixture was boiled
to remove 1,800 ml of solvent by distillation and thereafter 1,000 ml of
freshly distilled tetrahydrofuran was added.
Boiling was continued under reflux for a period of 16 hours. The solution was
then concentrated to about one-half its original volume by distillation and was
poured slowly with vigorous stirring into a large volume of ice water
containing 340 g of ammonium chloride. The aqueous solution was saturated
with sodium chloride and extracted with benzene. The benzene extract was
washed with saturated brine, and both aqueous layers were washed again
with the same portions of benzene.
The combined benzene layers were dried over anhydrous sodium carbonate
and the solvent was removed at reduced pressure to give 90.5 g of crude
crystalline 5α,17α-dihydroxy-6β-methylallopregnane-3,20-dione 3,20-bis-
(ethylene ketal). Half of the residue, 45.2 g, was recrystallized from acetone
and some methylene chloride to give 34.4 g of 5α,17α-dihydroxy-6βmethylallopregnane-3,20-dione 3,20-bis-(ethylene ketal). A sample
recrystallized from acetone and methylene chloride for analysis melted at
160° to 163°C.
Preparation of 5α,17α-Dihydroxy-6β-Methylallopregnane-3,20-dione: A
solution was prepared containing 38.9 g of 5α,7α-dihydroxy-6βmethylallopregnane-3,20-dione 3,20-bis-(ethylene ketal) in 389 ml of boiling
acetone. Thereto was added 39 ml of 1 N sulfuric acid in portions under
swirling and seeding with product. Boiling was continued for a period of 2
minutes and the mixture was allowed to stand at room temperature.
Thereafter the mixture was diluted with 1,500 ml of water, chilled and filtered.
The precipitate was washed with water, dilute ammonium hydroxide and
water, and dried in a vacuum oven overnight. The yield was 31.2 g which was
recrystallized by dissolving in 1,200 ml of dimethylformamide, heating to
150°C, cooling slightly, and adding 12 ml of hot water. The recrystallized
5α,17α-dihydroxy-6β-methylallopregnane-3,20-dione thus obtained was 28.75
g of MP 270° to 275.5°C. After an additional recrystallization from aqueous
dimethylformamide, the MP was 274° to 279°C.
Preparation of 6α-Methyl-17α-Hydroxyprogesterone: A suspension was made
by introducing 2 g of 5α,17α-dihydroxy-6β-methylallopregnane-3,20-dione
into 200 ml of chloroform. The suspension was chilled in an ice bath with
stirring, and thereupon hydrogen chloride was bubbled through the reaction
mixture for 80 minutes with continuous cooling and stirring. After bubbling in
nitrogen for a period of 15 minutes the solution was washed with water, 1 N
sodium bicarbonate solution and again with water.
The aqueous layers were rewashed with one portion of chloroform, and the
washings combined with the remainder of the chloroform solution. After
drying over anhydrous magnesium sulfate, the chloroform solution was
concentrated to dryness, then taken up in a small volume of methylene
chloride, treated with Magnesol anhydrous magnesium silicate and filtered.
Acetone was added to the solution and the solution was boiled to remove the
methylene chloride. After the solution was concentrated to a volume of about
15 ml it was chilled and the crystals were collected through filtration. The
1.37 g of crystals so obtained were recrystallized from acetone to give pure
6α-methyl-17α-hydroxyprogesterone of MP 220° to 223.5°C.
Preparation of 6α-Methyl-17-Hydroxyprogesterone 17-Acetate: 1 g of 6αmethyl-17α-hydroxyprogesterone was dissolved in a mixture of 10 ml of acetic
acid and 2 ml of acetic anhydride by heating. After solution was effected the
mixture was cooled to 15°C, and 0.3 g of p-toluenesulfonic acid was added.
After allowing the mixture to stand for a period of 2.5 hours at room
temperature, the pink solution was poured into ice water to give an
amorphous solid which was recovered by filtration.
The precipitate was washed carefully with water and was then dissolved in 10
ml of methanol and 1.5 ml of methylene chloride. The solution was concentrated to 10 ml, diluted with 0.5 ml of 10% sodium hydroxide, boiled
for one minute and cooled. The product, which crystallized on cooling, was
recrystallized to give flakes of 6α-methyl-17α-hydroxyprogesterone 17-
acetate, having a MP 205° to 209°C, according to US Patent 3,147,290.
brand name
Amen (Amarin); Curretab (Solvay Pharmaceuticals); Cycrin (ESI); Provera (Pharmacia & Upjohn).
Therapeutic Function
Progestin
Purification Methods
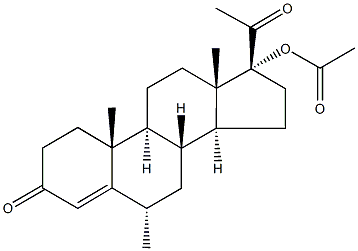
If it contains the epi-isomer (TLC), then dissolve it in CHCl3, bubble dry HCl gas to epimerise it, evaporate and recrystallise it from chloroform. The UV has max at 241nm ( 16,150) in EtOH. The 17-acetate [71-58-9] crystallises from MeOH with m 207-208o and [] D 25 +61o (CHCl3). Its UV has max at 240nm ( 15,950) in EtOH. [Babcock et al. J Am Chem Soc 80 2904 1958, Beilstein 8 IV 2212.]
MEDROXYPROGESTERONE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Wuhan Han Sheng New Material Technology Co.,Ltd | +8617798174412 | admin01@hsnm.com.cn | China | 2118 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29888 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8619521488211 | info@longyupharma.com | China | 2534 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569266 15319487004 | 1015@dideu.com | China | 4088 | 58 |
| Hebei Bonster Technology Co.,Limited | +8613315996897 | bsterltd.wendy@gmail.com | China | 796 | 58 |
| Hebei Runbin Biotechnology Co. LTD | 13180553332 | 2179877681@qq.com | CHINA | 996 | 58 |
| Longyan Tianhua Biological Technology Co., Ltd | 0086 18039857276 18039857276 | CHINA | 2783 | 58 |
View Lastest Price from MEDROXYPROGESTERONE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-18 | Medroxyprogesterone
520-85-4
|
US $0.00 / Kg/Bag | 1KG | 98%min HPLC | 100kgs | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2023-09-14 | MEDROXYPROGESTERONE
520-85-4
|
US $0.00-0.00 / KG | 1KG | 99% | 50TONS | Wuhan Han Sheng New Material Technology Co.,Ltd | |
 |
2023-08-21 | medroxyprogesterone
520-85-4
|
US $30.00 / kg | 1kg | 99% | 1000t/year | Anhui Ruihan Technology Co., Ltd |
-

- Medroxyprogesterone
520-85-4
- US $0.00 / Kg/Bag
- 98%min HPLC
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- MEDROXYPROGESTERONE
520-85-4
- US $0.00-0.00 / KG
- 99%
- Wuhan Han Sheng New Material Technology Co.,Ltd
-

- medroxyprogesterone
520-85-4
- US $30.00 / kg
- 99%
- Anhui Ruihan Technology Co., Ltd