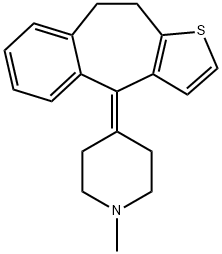
Pizotifen
- CAS No.
- 15574-96-6
- Chemical Name:
- Pizotifen
- Synonyms
- Litec;bc105;BC 105;PIZOTIFEN;Pizotifan;Pizotylene;Pizotyline;Pizotifenum;Sandomigram;Sandomigran
- CBNumber:
- CB4230373
- Molecular Formula:
- C19H21NS
- Molecular Weight:
- 295.44
- MDL Number:
- MFCD00864192
- MOL File:
- 15574-96-6.mol
- MSDS File:
- SDS
| Melting point | 140-142°C |
|---|---|
| Boiling point | 436.7±45.0 °C(Predicted) |
| Density | 1.164±0.06 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO: ≥8mg/mL |
| pka | pKa 6.95 (Uncertain) |
| form | powder |
| color | White to Off-White |
| Merck | 14,7515 |
| CAS DataBase Reference | 15574-96-6(CAS DataBase Reference) |
| FDA UNII | 0BY8440V3N |
| ATC code | N02CX01 |
| NIST Chemistry Reference | Pizotyline(15574-96-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H361 | |||||||||
| Precautionary statements | P201-P301+P312+P330-P308+P313 | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 36/37/38-22-63 | |||||||||
| Safety Statements | 26-37/39-36/37 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TM7165000 | |||||||||
| HS Code | 2934.99.3000 | |||||||||
| NFPA 704 |
|
Pizotifen price More Price(28)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | B9688 | Pizotifen ≥98% (HPLC) | 15574-96-6 | 10mg | $98.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | B9688 | Pizotifen ≥98% (HPLC) | 15574-96-6 | 50mg | $390 | 2024-03-01 | Buy |
| TCI Chemical | P2344 | Pizotifen >98.0%(HPLC) | 15574-96-6 | 50mg | $42 | 2021-12-16 | Buy |
| TCI Chemical | P2344 | Pizotifen >98.0%(HPLC) | 15574-96-6 | 200mg | $109 | 2024-03-01 | Buy |
| TRC | P552800 | Pizotyline | 15574-96-6 | 50mg | $65 | 2021-12-16 | Buy |
Pizotifen Chemical Properties,Uses,Production
Antihistamine
Pizotyline is also referred to as pizotifene and pizotifen malate. It is a chemical synthetic antihistamine. Its chemical structure is similar to that of heptaidime and amitriptyline. It has a strong anti 5serotonin and antihistamine effect and weak anti acetylcholine action, which is one of the commonly used drugs to prevent migraine. This product also inhibits the analgesic effect of bradykinin (BK) on the peripheral nerve and its sedative and antidepressant effects. This product can reduce the tolerance to ethanol, and can strengthen the effect of diazepam, sedative and tricyclic antidepressant. It is clinically used in typical and atypical migraine. It can reduce symptoms, reduce the number of episodes and duration, and the effect is significant. But it has no immediate effect on the acute attack of migraine. It can be used in erythromelalgia, angioedema, chronic urticaria, skin scratch disease etc.. It has also been reported that pizotyline can be used for the treatment of the carcinoid syndrome caused diarrhea, facial flushing and carotid artery pain as well as polycythemia induced pruritus.
Physicochemical properties
It is white needle like crystal, odorless, with bitter taste. The melting point is 147.5 151.5 centigrade. It can dissolve in organic solvent or acid solution, such as ethanol, chloroform, etc., insoluble in water. Pizotyline is synthesized by the multistep reaction using 2chloromethyl thiophene as raw material. It's an analgesic and mainly used for the treatment of typical and atypical migraine. It can also be used for chronic urticaria, atrial and ventricular premature beat.
Instruction
- The drug is of small toxicity and can be used for a long-term; After six months of continuous medication, the patients can stop the use to observe the effect of this drug and avoid the accumulation of drugs in the body.
- Attention should be paid to change of blood image in long term use.
- Pizotyline can not be used in combination with monoamine oxidase inhibitors.
Precaution
- This product can cause lethargy, fatigue, increased appetite, and weight gain with rare nausea, dizziness, flushing, muscle pain, etc.. Generally, they are common in the beginning medication of 1~2 weeks, and they will gradually reduce or disappear in the continued medication.
- It is prohibited for patients with angle closure glaucoma or prostate hypertrophy dysuria
- Motor vehicle drivers and high-altitude operations should be cautious to take pizotyline following the instructions of doctors.
Adverse reaction
- Drowsiness and hyperactivity: somnolence is commonly seen within 1~2 weeks of starting medication, and it can gradually decrease or disappear after taking medicine, and the body weight gradually stabilizes after 6 months. The driver, the air operator should be cautious in the drug taking.
- Other side effects: muscle soreness or painful spasms, restless legs, fluid retention, mild headache, vertigo, flushing, low sexual desire, exacerbation of epilepsy, dreaminess, palpitation, rash, menstrual disorder, insomnia and leukocyte decline.
- Anticholinergic action: it is forbidden for patients with glaucoma, prostatic hypertrophy and pregnant women. In addition, patients with long-term use of pizotifen should pay attention to changes in blood.
Chemical Properties
White to Off-White Solid
Originator
Sandomigran ,Sandoz ,Italy ,1972
Uses
benzocycloheptane based drug
Uses
Serotonin antagonist structurally related to Cyproheptadine. Antimigraine; appetite stimulant.
Indications
Pizotifen, a potent antihistamine and antiserotonin agent usually prescribed for migraine prophylaxis, has had reported success in patients with polycythemia vera.
Definition
ChEBI: A benzocycloheptathiophene that is 9,10-dihydro-4H-benzo[4,5]cyclohepta[1,2-b]thiophene 4-ylidene)-1-methylpiperidine which is joined from the 4 position to the 4 position of an N-methylpiperidine moiety b a double bond. It is a sedating antihistamine, with strong serotonin antagonist and weak antimuscarinic activity. It is generally used as the malate salt for the treatment of migraine and the prevention of headache attacks during cluster periods.
Manufacturing Process
(A) Preparation of Thenylidene-(2)-Phthalide: 24.2 g of thienyl-(2)-acetic acid, 52.0 g of phthalic acid anhydride, 4.0 g of anhydrous sodium acetate and 125 ml of 1-methylpyrrolidone-(2) are heated while stirring in an open flask for 3 hours to 205° to 208°C, while nitrogen is passed through. It is then cooled and the viscous reaction mixture poured into 1 liter of water. The precipitated substance is filtered off, washed with water and then dissolved in 200 ml of chloroform. After filtering off some undissolved substance, shaking is effected twice with 100 ml of 2 N sodium carbonate solution and then with water, drying is then carried out over sodium sulfate and the volume is reduced by evaporation. The crude phthalide is repeatedly recrystallized from ethanol, while treating with animal charcoal. It melts at 114° to 115°C.
(B) Preparation of o-[2-Thienyl-(2')-Ethyl]Benzoic Acid: 24.0 g of thenylidene(2)-phthalide, 8.8 g of red pulverized phosphorus, 240 ml of hydrochloric acid (d = 1.7) and 240 ml of glacial acetic acid are heated to boiling under nitrogen and while stirring vigorously. 70 ml toluene are then added and 6.0 g of red phosphorus added in small portions over a period of 1 hour. It is then poured into 3 liters of ice water, stirred with 300 ml of chloroform and the phosphorus removed by filtration.
The chloroform phase is then removed, the aqueous phase extracted twice more with 200 ml of chloroform and the united extracts shaken out 4 times,each time with 200 ml of 2 N sodium hydroxide solution. The alkaline solution is then rendered acid to Congo red reagent, using hydrochloric acid and extracted 3 times with chloroform. After drying over sodium sulfate and evaporating the solvent, the residue is chromatographed on aluminum oxide (Activity Stage V). The substance eluted with benzene and benzene/chloroform (1:1) is recrystallized from chloroform/hexane (1:1); MP 107° to 109°C.
(C) Preparation of 9,10-Dihydro-4H-Benzo[4,5]Cyclohepta[1,2-b]Thiophen(4)-One: 200 ml of 85% phosphoric acid and 112 g of phosphorus pentoxide are heated to 135°C. 7.0 g of o-[2-thienyl-(2')-ethyl]benzoic acid are then introduced while stirring thoroughly over a period of 30 min. Stirring is then continued for another hour at 135°C and the reaction mixture is then stirred into 1 liter of ice water. Extraction is then effected 3 times, using 250 ml ether portions, the ethereal extract is washed with 2 N sodium carbonate solution, dried over sodium sulfate and reduced in volume by evaporation. The residue is boiled up with 55 ml of ethanol, the solution freed of resin by decanting and then stirred at room temperature for 6 hours with animal charcoal. It is then filtered off, reduced in volume in a vacuum and the residue distilled. BP 120° to 124°C/0.005 mm, nD24.5 = 1.6559.
(D) Preparation of 4-[1'-Methyl-Piperidyl-(4')]-9,10-Dihydro-4HBenzo[4,5]Cyclohepta[1,2b]Thiophen-(4)-ol: 0.94 g of magnesium filings which have been activated with iodine are covered with a layer of absolute tetrahydrofuran and etched with a few drops of ethylene bromide. A solution of 5.0 g of 1-methyl-4-chloropiperidine in 5 ml of tetrahydrofuran is then added dropwise and boiling then effected for a further hour under reflux. After cooling to room temperature, the solution of 4.5 g of 9,10-dihydro-4Hbenzo[4,5]cyclohepta[1,2-b]thiophen-(4)-one in 5 ml of tetrahydrofuran is added dropwise.
Stirring is carried out first for 3 hours at room temperature and then for 2 hours at boiling temperature, it is then cooled and poured into 300 ml of icecold 20% ammonium chloride solution. It is then shaken out with methylene chloride, the methylene chloride solution washed with water and shaken 3 times with 30 ml portions of aqueous 2 N tartaric acid solution. The tartaric acid extract is rendered alkaline while cooling thoroughly and then extracted twice with methylene chloride. After washing with water, drying over potassium carbonate and reducing in volume by evaporation, the residue is recrystallized from ethanol. MP 197° to 199°C.
(E) Preparation of 4-[1'-Methyl-Piperidylidene-(4')]-9,10-Dihydro-4HBenzo[4,5]Cyclohepta[1,2-b]Thiophene Hydrochloride: 2 g of 4-[1'-methylpiperidyl-(4')]-9,10-dihydro4H-benzo[4,5]cyclohepta[1,2-b]thiophen-(4)-ol, 60 ml of glacial acetic acid and 20 ml of concentrated hydrochloric acid are boiled for 30 minutes under reflux. After evaporating in a vacuum, the residue is triturated with 3 ml of acetone, the precipitated hydrochloride is then filtered off and it is recrystallized from isopropanol/ether. MP 261° to 263°C (decomposition).
brand name
Sandomigran (Novartis).
Therapeutic Function
Migraine therapy
Biochem/physiol Actions
Pizotifen is a serotonin antagonist acting mainly at the 5-HT1, 5-HT2A and 5HT2C receptors with some antihistamine activity. It is used for the prevention of vascular headache including migraine and cluster headache.
Clinical Use
Prophylactic treatment of vascular headaches including migraine
Drug interactions
Potentially hazardous interactions with other drugs
Adrenergic neurone blockers: pizotifen antagonises
hypotensive effect.
Metabolism
Pizotifen undergoes extensive metabolism.
Over half of a dose is excreted in the urine, chiefly as
metabolites; a significant proportion is excreted in the
faeces.
Pizotifen Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Xiamen Wonderful Bio Technology Co., Ltd. | +8613043004613 | Sara@xmwonderfulbio.com | China | 305 | 58 |
| QINGDAO HONG JIN CHEMCIAL CO.,LTD. | 532-83657313 | hjt@hong-jin.com | China | 228 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 | 1056@dideu.com | China | 3581 | 58 |
| WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD | +8615377521700 | wangwendy93@gmail.com | China | 868 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
View Lastest Price from Pizotifen manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-16 | Pizotifen
15574-96-6
|
US $0.00 / KG | 100g | 98%+ | 100kg | WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD | |
 |
2024-01-18 | Pizotifen
15574-96-6
|
US $88.00 / KG | 1KG | 99% | 5000 | Xiamen Wonderful Bio Technology Co., Ltd. | |
 |
2023-09-06 | Pizotifen
15574-96-6
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd |