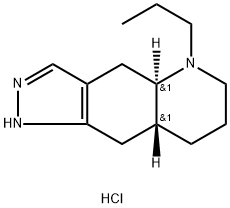
(-)-QUINPIROLE HYDROCHLORIDE
- CAS No.
- 85798-08-9
- Chemical Name:
- (-)-QUINPIROLE HYDROCHLORIDE
- Synonyms
- LY-171,555;()-Quinpirole;(-)-LY 171555;(4ar-trans)-lorid;(-)-QUINPIROLE HCL;(-)-Quinpirole HCl;(-)-QUINPIROLE HYDROCHLORIDE;(-)-QUINPIROLE HYDROCHLORIDE;(-)-quinpirole monohydrochloride;(-)-QUINPIROLE HYDROCHLORIDE (LY-171555) SELECTIVE D2 DOPAMINE
- CBNumber:
- CB4687090
- Molecular Formula:
- C13H22ClN3
- Molecular Weight:
- 255.79
- MDL Number:
- MFCD00069336
- MOL File:
- 85798-08-9.mol
- MSDS File:
- SDS
| storage temp. | -20°C |
|---|---|
| solubility | 0.1 M HCl: soluble23mg/mL |
| form | solid |
| color | white |
| optical activity | [α]25/D 124.5°, c = 0.4 in H2O(lit.) |
| Stability | Hygroscopic |
| FDA UNII | T6I2W5V2K1 |
(-)-QUINPIROLE HYDROCHLORIDE price More Price(14)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | Q102 | (?)-Quinpirole hydrochloride ≥98% (HPLC), solid | 85798-08-9 | 10mg | $158 | 2024-03-01 | Buy |
| Sigma-Aldrich | Q102 | (?)-Quinpirole hydrochloride ≥98% (HPLC), solid | 85798-08-9 | 25mg | $436 | 2024-03-01 | Buy |
| Sigma-Aldrich | 5.05982 | (-)-Quinpirole Hydrochloride - CAS 85798-08-9 - Calbiochem | 85798-08-9 | 10mg | $184 | 2022-05-15 | Buy |
| Sigma-Aldrich | Q102 | (?)-Quinpirole hydrochloride ≥98% (HPLC), solid | 85798-08-9 | 100mg | $1480 | 2024-03-01 | Buy |
| TRC | Q796713 | (-)-QuinpiroleHydrochloride | 85798-08-9 | 5mg | $250 | 2021-12-16 | Buy |
(-)-QUINPIROLE HYDROCHLORIDE Chemical Properties,Uses,Production
Uses
Antihypertensive.
Uses
(-)-Quinpirole hydrochloride has been used as a selective D2 dopamine (DA) receptor agonist in various experiments.
Biological Activity
(-)-quinpirole hydrochloride is an agonist of dopamine d2-like receptor [1].dopamine d2-like receptor reduces neuron’s excitability after activation by dopamine, making it an important drug target in schizophrenia and parkinson’s disease, but its ligands also cause cognitive inflexibility such as poor reversal learning [1, 2].in spiny neurons, (-)-quinpirole hydrochloride (5 ~ 10 μm) markedly reduced evoked firing in area x and lobus parolfactorius (lpo). unlike the enhancement of excitability by dopamine d1 receptor activation, the reduction in evoked firing by (-)-quinpirole hydrochloride was not voltage-dependent. this effect was antagonized by the dopamine d2-like receptor antagonist sulpiride (10 μm), which suggested that the effect of (-)-quinpirole hydrochloride was specific to dopamine d2-like receptor [2].in rats, (-)-quinpirole hydrochloride (0.01, 0.025, 0.1, 0.25, and 0.5 mg/kg) impaired both visual reversal learning task and spatial probabilistic reversal learning (prl) task in a dose-dependent manner. in analysis of the probe trials on the visual task, (-)-quinpirole hydrochloride at 0.25 mg/kg induced a complete blockade of learning from negative feedback, whilst learning from positive feedback was intact. estimated parameters from the model that best described the prl choice data revealed a steep and selective decrease in learning rate from losses [1].[1]. alsiö j, phillips b u, sala-bayo j, et al. dopamine d2-like receptor stimulation blocks negative feedback in visual and spatial reversal learning in the rat: behavioural and computational evidence. psychopharmacology (berl), 2019, 236(8): 2307-2323.[2]. ding l, perkel d j. dopamine modulates excitability of spiny neurons in the avian basal ganglia. the journal of neuroscience, 2002, 22(12): 5210-5218.
Biochem/physiol Actions
Quinpirole is a dopamine agonist with high affinity for the D2 and D3 dopamine receptor subtypes. Specific [3H]quinpirole binding in rat brain was saturable, and dependent on temperature, membrane concentration, sodium concentration and guanine nucleotides. The putative D2 dopamine receptor agonist quinpirole (LY 171,555) is the most widely used D2 agonist in in vivo and in vitro studies. Quinpirole hydrochloride is an active enantiomer of (±)-quinpirole.Saturation analysis revealed high affinity binding characteristics (KD = 2.3 +/- 0.3 nM) which were confirmed by association-dissociation kinetics. The regional distribution of [3H]quinpirole binding sites roughly paralleled the distribution of [3H]spiperone binding sites, with greatest densities present in the striatum, nucleus accumbens and olfactory tubercles. A variety of drugs, most notably monoamine oxidase inhibitors (MAOls), inhibit the binding of [3H]quinpirole, but not [3H]spiperone or [3H](-)N-n-Propylnorapomorphine, in rat striatal membranes by a mechanism that does not appear to involve the enzymatic activity of MAO. Clinically antidepressant MAOIs exhibited selectivity between sites labeled by [3H]quinpirole and [3H]spiperone as did a number of structurally related propargylamines and N-acylethylenediamine derivatives and other drugs such as debrisoquin and phenylbiguanide. The MAOIs clorgyline and Ro 41-1049 were the most potent. MAOIs interact with a novel binding site that is labeled by [3H]quinpirole or that modulates [3H]quinpirole binding. This site may be associated with D2-like dopamine receptors.
storage
Desiccate at -20°C
(-)-QUINPIROLE HYDROCHLORIDE Preparation Products And Raw materials
Raw materials
Preparation Products
(-)-QUINPIROLE HYDROCHLORIDE Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6393 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | masar@topule.com | China | 8474 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| 3B Pharmachem (Wuhan) International Co.,Ltd. | 821-50328103-801 18930552037 | 3bsc@sina.com | China | 15848 | 69 |
| Sigma-Aldrich | 021-61415566 800-8193336 | orderCN@merckgroup.com | China | 51471 | 80 |
| EMMX Biotechnology LLC | 888-539-0666 | info@emmx.com | United States | 8449 | 60 |
| Shanghai EFE Biological Technology Co., Ltd. | 021-65675885 18964387627 | info@efebio.com | China | 9709 | 58 |
| Beijing OKA biological technology co., LTD | 010-62971590 18548936886 | 3462612863@qq.com | China | 6912 | 58 |
| BOC Sciences | -- | info@bocsci.com | USA | 0 | 65 |