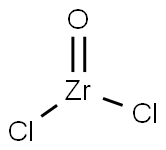
Zirconium oxychloride
- CAS No.
- 7699-43-6
- Chemical Name:
- Zirconium oxychloride
- Synonyms
- ZROCL2;ZIRCONIUM CHLORIDE;ZIRCONYL CHLORIDE;ZIRCONIUM DICHLORIDE OXIDE;nci-c60811;chlorozirconyl;JACS-7699-43-6;dichlorooxo-zirconiu;Dichlorooxozirconium;Zirconium oxychlorid
- CBNumber:
- CB6470074
- Molecular Formula:
- Cl2OZr
- Molecular Weight:
- 178.13
- MDL Number:
- MFCD00011309
- MOL File:
- 7699-43-6.mol
- MSDS File:
- SDS
| Melting point | −15 °C |
|---|---|
| Density | 1.344 g/mL at 25 °C |
| solubility | soluble in H2O, ethanol |
| form | Liquid |
| color | Clear colorless |
| Merck | 13,10237 |
| Stability | Stable. Incompatible with strong oxidizing agents, water, moisture, alkali metals. |
| CAS DataBase Reference | 7699-43-6(CAS DataBase Reference) |
| EWG's Food Scores | 1-3 |
| FDA UNII | T4294601O7 |
| EPA Substance Registry System | Zirconium, dichlorooxo- (7699-43-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|---|---|
| Signal word | Danger |
| Hazard statements | H290-H314-H335 |
| Precautionary statements | P234-P261-P271-P280-P303+P361+P353-P305+P351+P338 |
| Hazard Codes | C,Xn |
| Risk Statements | 23-34-37-20/21/22-36/38 |
| Safety Statements | 26-36-45-36/37/39-36/37 |
| RIDADR | UN 1789 |
| WGK Germany | 1 |
| HazardClass | 8 |
| PackingGroup | III |
Zirconium oxychloride price More Price(6)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 464198 | Zirconyl chloride solution 30% in hydrochloric acid | 7699-43-6 | 250ml | $263 | 2023-06-20 | Buy |
| American Custom Chemicals Corporation | ING0010511 | ZIRCONIUM OXYCHLORIDE 95.00% | 7699-43-6 | 10G | $1097.25 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | ING0010511 | ZIRCONIUM OXYCHLORIDE 95.00% | 7699-43-6 | 50G | $2512.13 | 2021-12-16 | Buy |
| Aaron Chemicals | AR005FXR | Zirconium,dichlorooxo- 36% | 7699-43-6 | 25g | $13 | 2021-12-16 | Buy |
| Aaron Chemicals | AR005FXR | Zirconium,dichlorooxo- 36% | 7699-43-6 | 100g | $32 | 2021-12-16 | Buy |
Zirconium oxychloride Chemical Properties,Uses,Production
Physical Properties
The octahydrate is a white silky solid; tetragonal crystals consisting of tetramers; effloresces; refractive index 1.552; loses six water molecules at 150°C; becomes anhydrous at 210°C; decomposes at 400°C; soluble in water; aqueous solution acidic; also soluble in alcohol and ether; slightly soluble in hydrochloric acid.

Uses
Zirconyl chloride is used to make pigment toners and improve properties of color lakes of acid and basic dyes. Also, it is used to prepare body deodorants and antiperspirant, water repellant, dye precipitant, catalysts, and many zirconium compounds.
Preparation
The compound is prepared by dissolving zirconium tetrachloride, ZrCl4, or sodium zirconate, Na4ZrO4, in hydrochloric acid solution followed by evaporation to obtain crystals of octahydrate.
Also, the compound can be prepared by reacting zirconium tetrachloride with chlorine oxide:
ZrCl4 + Cl2O → ZrOCl2 + 2Cl2
Toxicity
Moderately toxic by ingestion, intraperitoneal and subcutaneous routes.
LD50 (intraperitioneal) (rat): 400mg/kg
Chemical Properties
Zirconium oxychloride exists as white silky crystals, soluble in water and alcohol. With water, the octahydrate is formed as tetragonal white needles. It is extremely hygroscopic and decomposes to zirconium tetrachloride (ZrCl4) and zirconium oxide (ZrO2) at 250 C. It is also formed as the octahydrate [13520-92-8] as white, tetrahedral crystals that are very soluble in water, ethyl alcohol, ether, and methanol, among others, but only slightly soluble in hydrochloric acid. When heated to decomposition, it emits toxic fumes of hydrogen chloride.
Uses
Textile, cosmetic and grease additive, antiperspirant, water repellents, chemical reagent, zirconium salts, in lakes and toners of acidic and basic dyes, oil-field acidizing aid.
Uses
Building block for a new series of mixed zirconium phosphonates which may have uses in catalysis, ion exchange or photophysics.
Definition
ChEBI: Zirconyl chloride is a zirconium coordination entity consisting of zirconium(IV) bound to oxygen via a double bond and to two chlorines.
Reactivity Profile
Acidic salts, such as Zirconium oxychloride, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
Has only a mild pharmacological action. Inhalation of dust may irritate nose and throat. Contact with eyes or skin causes irritation.
Flammability and Explosibility
Not classified
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion and subcutaneous routes. Questionable carcinogen with experimental neoplastigenic data. When heated to decomposition it emits toxic fumes of Cl-. Used as an antiperspirant. See also ZIRCONIUM COMPOUNDS and CHLORIDES.
Purification Methods
Crystallise it repeatedly from 8M HCl to give ZrOCl2.8H2O (see below). On drying, ZrOCl2.6H2O, m 150o, is formed. The product is not free from hafnium. [Blumenthal J Chem Ed 39 607 1962.]
Zirconium oxychloride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12446 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| Hebei Shengyang Water Conservancy Engineering Co., Ltd. | +8615373025980 | clara@hbshengyang.com | China | 874 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18628 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21667 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2989 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8820 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
View Lastest Price from Zirconium oxychloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-05-10 | Zirconium oxychloride
7699-43-6
|
US $18.00-18.00 / kg | 1kg | 99.9 | 5000 | Hebei Shengyang Water Conservancy Engineering Co., Ltd. | |
 |
2024-04-28 | Zirconium oxychloride
7699-43-6
|
US $100.00 / kg | 1kg | 99 | 5000 | Hebei Zhuanglai Chemical Trading Co.,Ltd | |
 |
2024-04-10 | Zirconium oxychloride
7699-43-6
|
US $25.00-18.00 / kg | 1kg | 99.9% | 50000tons | Wuhan Boyuan Import & Export Co., LTD |
-

- Zirconium oxychloride
7699-43-6
- US $18.00-18.00 / kg
- 99.9
- Hebei Shengyang Water Conservancy Engineering Co., Ltd.
-

- Zirconium oxychloride
7699-43-6
- US $100.00 / kg
- 99
- Hebei Zhuanglai Chemical Trading Co.,Ltd
-

- Zirconium oxychloride
7699-43-6
- US $25.00-18.00 / kg
- 99.9%
- Wuhan Boyuan Import & Export Co., LTD
7699-43-6(Zirconium oxychloride)Related Search:
1of4