ChemicalBook >> CAS DataBase List >>ANTI-(1R)-(+)-CAMPHORQUINONE 3-OXIME
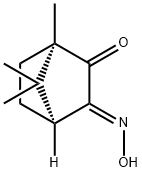
ANTI-(1R)-(+)-CAMPHORQUINONE 3-OXIME
- CAS No.
- 31571-14-9
- Chemical Name:
- ANTI-(1R)-(+)-CAMPHORQUINONE 3-OXIME
- Synonyms
- (1R,4S,E);-3-(Hydroxyimino);ISONITROSO-D-CAMPHOR;(1R,E)-(+)-CAMPHORQUINONE 3-OXIME;(1R,E)-(+)-2,3-BORNANEDIONE 3-OXIME;ANTI-(1R)-(+)-CAMPHORQUINONE 3-OXIME;ANTI-(1R)-(+)-2,3-BORNANEDIONE 3-OXIME;anti-(1R)-(+)-Camphorquinone 3-Oxime >ANTI-(1R)-(+)-CAMPHORQUINONE 3-OXIME, 99 %;(1R,E)-(+)-2,3-Bornanedione 3-oxime, anti-(1R
- CBNumber:
- CB7476402
- Molecular Formula:
- C10H15NO2
- Molecular Weight:
- 181.23
- MDL Number:
- MFCD00082864
- MOL File:
- 31571-14-9.mol
- MSDS File:
- SDS
Last updated:2023-06-08 17:06:36
| Melting point | 153-156 °C(lit.) |
|---|---|
| Boiling point | 276.8±7.0 °C(Predicted) |
| Density | 1.25±0.1 g/cm3(Predicted) |
| refractive index | 196 ° (C=1, EtOH) |
| storage temp. | 2-8°C |
| form | powder to crystal |
| pka | 9.35±0.60(Predicted) |
| color | White to Orange to Green |
| optical activity | [α]25/D +200°, c = 1 in ethanol |
| Sensitive | Air Sensitive |
| BRN | 3200377 |
SAFETY
Risk and Safety Statements
| Safety Statements | 22-24/25 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WGK Germany | 3 | |||||||||
| HS Code | 2928.00.5000 | |||||||||
| NFPA 704 |
|
ANTI-(1R)-(+)-CAMPHORQUINONE 3-OXIME price More Price(10)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 273112 | (1R,E)-(+)-Camphorquinone 3-oxime 99% | 31571-14-9 | 1g | $232 | 2023-06-20 | Buy |
| TCI Chemical | C1661 | anti-(1R)-(+)-Camphorquinone 3-Oxime >95.0%(GC)(N) | 31571-14-9 | 1g | $121 | 2024-03-01 | Buy |
| Alfa Aesar | H51034 | (1R,E)-(+)-Camphorquinone 3-oxime, 99% | 31571-14-9 | 5g | $473 | 2023-06-20 | Buy |
| Alfa Aesar | H51034 | (1R,E)-(+)-Camphorquinone 3-oxime, 99% | 31571-14-9 | 1g | $118 | 2021-12-16 | Buy |
| TRC | C175015 | anti-(1R)-(+)-Camphorquinone 3-Oxime | 31571-14-9 | 100mg | $90 | 2021-12-16 | Buy |
ANTI-(1R)-(+)-CAMPHORQUINONE 3-OXIME Preparation Products And Raw materials
Raw materials
Preparation Products
ANTI-(1R)-(+)-CAMPHORQUINONE 3-OXIME Suppliers
Global( 43)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 | peter68@ptchemgroup.com | China | 35453 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 50000 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 11013 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131981 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 94838 | 76 |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 021-61259108 18621169109 | market03@meryer.com | China | 40240 | 62 |
| Alfa Aesar | 400-6106006 | saleschina@alfa-asia.com | China | 30132 | 84 |
| TCI (Shanghai) Development Co., Ltd. | 021-67121386 | Sales-CN@TCIchemicals.com | China | 24539 | 81 |
31571-14-9(ANTI-(1R)-(+)-CAMPHORQUINONE 3-OXIME)Related Search:
DL-CAMPHORIC ANHYDRIDE
(+/-)-EXO EXO-2 3-CAMPHANEDIOL
(+)-10-CAMPHORSULFONIMINE
(-)-(8,8-DICHLOROCAMPHORYLSULFONYL)OXAZIRIDINE
(1R)-(-)-10-Camphorsulfonic acid
L(-)-10-Camphorsulfonyl chloride
Rucaparib Camsylate
D-CAMPHOR
(1S)-(+)-Camphor-10-sulphonic acid
(+)-(8,8-DICHLOROCAMPHORYLSULFONYL)OXAZIRIDINE
1of4
(1R,E)-(+)-2,3-BORNANEDIONE 3-OXIME
(1R,E)-(+)-CAMPHORQUINONE 3-OXIME
ANTI-(1R)-(+)-2,3-BORNANEDIONE 3-OXIME
ANTI-(1R)-(+)-CAMPHORQUINONE 3-OXIME
ISONITROSO-D-CAMPHOR
ANTI-(1R)-(+)-CAMPHORQUINONE 3-OXIME, 99 %
(1R,E)-(+)-2,3-Bornanedione 3-oxime, anti-(1R
(1R,E)-(+)-2,3-Bornanedione 3-oxime, anti-(1R)-(+)-Camphorquinone 3-oxime
anti-(1R)-(+)-Camphorquinone 3-Oxime
(1R,4S,E)
-3-(Hydroxyimino)
(2Z)-2-hydroxyimino-4,7,7-trimethylbicyclo[2.2.1]heptan-3-one
Bicyclo[2.2.1]heptane-2,3-dione,1,7,7-trimethyl-, 3-oxime, (1R,3E,4S)-
anti-(1R)-(+)-Camphorquinone 3-Oxime >
(Z)-3-(hydroxyimino)-1,7,7-trimethylbicyclo[2.2.1]Heptan-2-one
31571-14-9
Bicyclic Monoterpenes
Terpenes
Organic Building Blocks
Oximes
Asymmetric Synthesis
Chiral Building Blocks
Bicyclic Monoterpenes
Biochemistry
Terpenes