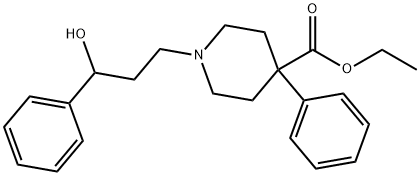
phenoperidine
- CAS No.
- 562-26-5
- Chemical Name:
- phenoperidine
- Synonyms
- phenoperidine;IPOPQVVNCFQFRK-UHFFFAOYSA-N;ethyl 1-(3-hydroxy-3-phenylpropyl)-4-phenylpiperidine-4-carboxylate;1-(3-Hydroxy-3-phenylpropyl)-4-phenylpiperidine-4-carboxylic acid ethyl ester;4-Piperidinecarboxylic acid, 1-(3-hydroxy-3-phenylpropyl)-4-phenyl-, ethyl ester
- CBNumber:
- CB7930605
- Molecular Formula:
- C23H29NO3
- Molecular Weight:
- 367.48
- MDL Number:
- MOL File:
- 562-26-5.mol
| Boiling point | 508.8±50.0 °C(Predicted) |
|---|---|
| Density | 1.122±0.06 g/cm3(Predicted) |
| pka | 14.31±0.20(Predicted) |
| FDA UNII | G9BH09J4JW |
| ATC code | N01AH04 |
SAFETY
Risk and Safety Statements
| RIDADR | 3249 |
|---|---|
| HazardClass | 6.1(b) |
| PackingGroup | III |
| DEA Controlled Substances | CSCN: 9641 CSA SCH: Schedule I NARC: Yes |
phenoperidine Chemical Properties,Uses,Production
Originator
Operidine ,Janssen ,US ,1965
Definition
ChEBI: Phenoperidine is a member of piperidines.
Manufacturing Process
The starting materials for the overall process are phenylacetonitrile with bischloroethyl toluene sulfonyl amide. These react to give a product which hydrolyzes to normeperidine (4-carboethoxy-4-phenylpiperidine). Condensation of that material with benzoylethylene gives the ketone: β-(4carboethoxy-4-phenylpiperidino)propiophenone.
A reaction mixture was prepared containing 4 grams of β-(4-carboethoxy-4phenylpiperidino)-propiophenone hydrochloride, 100 ml of methanol and about 0.5 gram of platinum oxide catalyst. The mixture was placed in a low pressure hydrogenation apparatus and was hydrogenated at a temperature of about 27°C and a pressure of about 3.5 atmospheres of hydrogen to convert the keto group of the β-(4-carboethoxy-4-phenylpiperidino)-propiophenone to a hydroxy group, and to form 3-(4-carboethoxy-4-phenylpiperidino)-1-phenyl-1propanol hydrochloride. After the hydrogenation was complete, the catalyst was separated from the reaction mixture by filtration, and the filtrate was evaporated to dryness in vacuo leaving a residue containing 3-(4carboethoxy-4-phenylpiperidino)-1-phenyl-l-propanol hydrochloride. The residue was digested with ethyl acetate thereby causing 3-(4-carboethoxy-4phenylpiperidino)-1-phenyl-1-propanol hydrochloride to crystallize. This compound melted at about 188°-189°C after being recrystallized three times from an ethyl acetate-methanol solvent mixture, according to US Patent 2,951,080.
Therapeutic Function
Analgesic