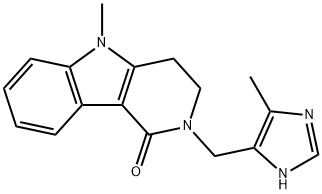
ALOSETRON
- CAS No.
- 122852-42-0
- Chemical Name:
- ALOSETRON
- Synonyms
- ALOSETRON;GR-68755X;GR-68755;ALOSETRON USP/EP/BP;Alosetron (GR-68755X;GR 68755; GR 68755X; LOTRONEX;GR-68755;GR68755;9-methyl-3-(5-methyl-3H-Imidazol-4-ylmethyl)- 1,2,3,9-Tetrahydro-;5-METHYL-2-[(5-METHYL-1H-IMIDAZOL-4-YL)METHYL]-3,4-DIHYDROPYRIDO[4,3-B]INDOL-1-ONE;9-methyl-3-(5-methyl-3H-Imidazol-4-ylmethyl)-1,2,3,9-Tetrahydro-Carbazol-4-One Hydrochloride;5-Methyl-2-[(4-methyl-1H-imidazol-5-yl)methyl]-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indol-1-one;1H-Pyrido[4,3-b]indol-1-one, 2,3,4,5-tetrahydro-5-methyl-2-[(4-methyl-1H-imidazol-5-yl)methyl]-
- CBNumber:
- CB8149787
- Molecular Formula:
- C17H18N4O
- Molecular Weight:
- 294.35
- MDL Number:
- MFCD00865546
- MOL File:
- 122852-42-0.mol
| Melting point | 238-240 °C |
|---|---|
| Boiling point | 648.1±55.0 °C(Predicted) |
| Density | 1.34±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO |
| form | Powder |
| pka | 14.10±0.10(Predicted) |
| FDA UNII | 13Z9HTH115 |
| ATC code | A03AE01 |
ALOSETRON price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| ApexBio Technology | A3157 | Alosetron | 122852-42-0 | 10mg | $49 | 2021-12-16 | Buy |
| ChemScene | CS-0640 | Alosetron | 122852-42-0 | 5mg | $55 | 2021-12-16 | Buy |
| ChemScene | CS-0640 | Alosetron | 122852-42-0 | 10mg | $85 | 2021-12-16 | Buy |
| ApexBio Technology | A3157 | Alosetron | 122852-42-0 | 50mg | $150 | 2021-12-16 | Buy |
| ChemScene | CS-0640 | Alosetron | 122852-42-0 | 25mg | $150 | 2021-12-16 | Buy |
ALOSETRON Chemical Properties,Uses,Production
Chemical Properties
crystalline powder
Originator
Alosetron hydrochloride,GlaxoSmithKline
Uses
Anti-emetic.
Definition
ChEBI: A pyrido[4,3-b]indole compound having a 5-methyl-1H-imidazol-4-ylmethyl group at the 2-position.
Indications
Alosetron (Lotronex) is a 5-HT3 receptor antagonist. Blocking this receptor results in decreased GI motility. Alosetron received FDA approval in February 2000 for the treatment of women with diarrhea-predominant IBS. In November 2000, at the request of the FDA, the drug was voluntarily withdrawn due to reported cases of ischemic colitis, including some fatalities.
Manufacturing Process
4-(Chloromethyl)-1-(triphenylmethyl)-1H-imidazole.
Thionyl chloride (0.829 g) was added over 1 min to a stirred suspension of 1-
(triphenylmethyl)-1H-imidazole-4-methanol (1.3 g) in a mixture of
dichloromethane (50 ml) and DMF (1.0 ml) at 23°C. The solution so obtained
was stirred for 15 min. and extracted with 8% sodium bicarbonate solution
(80 ml). The organic phase was washed with water (50 ml), dried and
evaporated to give an oil which solidified. The solid was slurried in hexane and
filtered to give the title compound (1.28 g), m.p. 139-141°C.
3,4-Dihydro-4-methylcyclopent[b]indol-1(2H)-one oxime.
3,4-Dihydro-4-methylcyclopent[b]indol-1(2H)-one (1.7 g) and hydroxylamine
hydrochloride (1.925 g) in pyridine were heated at 60°C for 18 h and cooled.
The reaction mixture was evaporated in vacuo to a residue to which was
added 8% sodium bicarbonate (150 ml). Extraction with ethyl acetate (300
ml) produced a suspension in the organic layer; this layer and associated solid
was separated from the aqueous layer. The aqueous layer was re-extracted
with ethyl acetate (250 ml). The combined organic extracts (and suspended
solid) were evaporated to a residue, boiled with a mixture of ethanol (150 ml)
and methanol (150 ml) and cooled to 50°C. The residue was adsorbed from
this solution on to FCC silica and applied to an FCC column. Elution with ethyl
acetate/3-10% methanol provided the title compound (1.69 g), m.p. 219-
224°C (decomp.).
2,3,4,5-Tetrahydro-5-methyl-1H-pyrido[4,3-b]indol-1-one.
3,4-Dihydro-4-methylcyclopent[b]indol-1(2H)-one oxime (1.53 g),
polyphosphoric acid (409 g) and dioxan (15 ml) were heated at 110-120°C for
2.2 h under nitrogen. The reaction mixture was cooled, and treated with 2 N
sodium carbonate solution (1 L). The suspension was extracted with ethyl
acetate (4x400 ml) and the combined extracts were dried. Evaporation gave a
solid (1.43 g) which was recrystallised from ethyl acetate/cyclohexane. This
solid was purified by FCC, eluting with dichloromethane:ethanol:ammonia
solution (200:10:1) to give a solid (1.26 g) which was recrystallised from
ethanol to provide the title compound (960 mg), m.p. 234-238°C.
2,3,4,5-Tetrahydro-5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-1Hpyrido[
4,3-b]indol-1-one maleate.
A mixture of 2,3,4,5-tetrahydro-5-methyl-1H-pyrido[4,3-b]indol-1-one (0.6 g)
and 78% sodium hydride dispersion in mineral oil (0.109 g) in dry DMF (15
ml) was stirred under nitrogen at 50°C until hydrogen evolution ceased (ca.
1.5 h). The mixture was cooled to 40°C and a solution of 4-(chloromethyl)-5-
methyl-1-(triphenylmethyl)-1H-imidazole (1.12 g) in dry THF (15 ml) was
added. The reaction was then stirred at 40°C for 3 h, at 20°C for 16 h and a
further portion of 4-(chloromethyl)-5-methyl-1-(triphenylmethyl)-1H-imidazole
(1.12 g) in dry THF (15 ml) was added. The resulting mixture was heated at
40°C for 3 h, quenched with water (20 ml) and acetic acid (20 ml), and
heated at 100°C for 2 h. The mixture was then concentrated in vacuo to ca.
60 ml, diluted with 1 M hydrochloric acid (40 ml) and washed with ethyl
acetate (3x50 ml). The organic phase was discarded and the acidic aqueous
phase was basified (pH=9) with potassium carbonate and extracted with ethyl
acetate:ethanol (20:1; 3x100 ml). The extracts were combined, dried and
evaporated to give a brown gum (ca. 1 g). This gum was adsorbed onto silica
and purified by FCC eluting with dichloromethane:ethanol:ammonia solition
(100:8:1) to give a pale brown solid (0.8 g); m.p. 238-240°C (decomp.). This
solid was dissolved in a mixture of (hot ethanol and methanol (1:1; 100 ml)
and treated with an ethanolic solution of maleic acid (3.18 g). The resulting
solution was concentrated to ca. 20 ml and diluted with dry diethyl ether (ca.
8 ml) to precipitate the title compound (0.75 g) as an off-white solid melting
point 160-162°C. Hydrochloride may be prepared by treating the above solid
with an equivalent of an ethanolic solution of HCl.
brand name
Lotronex (GlaxoSmithKline).
Therapeutic Function
Antidiarrheal
ALOSETRON Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6313 | 58 |
| Xi'an MC Biotech, Co., Ltd. | 029-89275612 +8618991951683 | mcbio_sales@163.com | China | 2255 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9271 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29067 | 58 |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 | gksales1@gk-bio.com | China | 9339 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418684 +8618949823763 | sales@tnjchem.com | China | 25363 | 58 |
View Lastest Price from ALOSETRON manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-08-04 | ALOSETRON USP/EP/BP
122852-42-0
|
US $1.10 / g | 1g | 0.999 | 100 Tons min | Dideu Industries Group Limited | |
 |
2021-07-13 | Alosetron
122852-42-0
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2021-07-09 | Alosetron
122852-42-0
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- ALOSETRON USP/EP/BP
122852-42-0
- US $1.10 / g
- 0.999
- Dideu Industries Group Limited
-

- Alosetron
122852-42-0
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- Alosetron
122852-42-0
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd