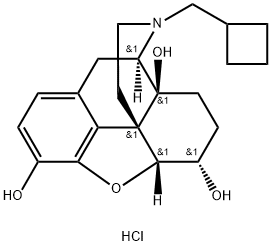
NALBUPHINE HYDROCHLORIDE
- CAS No.
- 23277-43-2
- Chemical Name:
- NALBUPHINE HYDROCHLORIDE
- Synonyms
- nubain;en2234a;6-alpha)-lph;NalbuphineHCl;nalbufinaclorhidrato;Nabrone hydrochloride;Emorfazone Impurity 4;NALBUPHINE HYDROCHLORIDE;YZLZPSJXMWGIFH-BCXQGASESA-N;NALBUPHINE HYDROCHLORIDE HYDRATE
- CBNumber:
- CB8750372
- Molecular Formula:
- C21H28ClNO4
- Molecular Weight:
- 393.91
- MDL Number:
- MFCD00069325
- MOL File:
- 23277-43-2.mol
| Flash point | 9℃ |
|---|---|
| storage temp. | -20°C |
| solubility | H2O: soluble |
| FDA UNII | ZU4275277R |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H300+H310+H330 |
| Precautionary statements | P260-P262-P264-P280-P302+P352+P310-P304+P340+P310 |
| Hazard Codes | T+,T,F |
| Risk Statements | 26/27/28-39/23/24/25-23/24/25-11 |
| Safety Statements | 22-36/37/39-45-36/37-16 |
| RIDADR | 1544 |
| WGK Germany | 3 |
| RTECS | QD3181000 |
| HazardClass | 6.1(a) |
| PackingGroup | II |
NALBUPHINE HYDROCHLORIDE Chemical Properties,Uses,Production
Originator
Nubain,Du Pont,US,1979
Uses
Nalbuphine is the free base form of Nalbuphine Hydrochloride. Nalbuphine is a mixed opioid agonist-antagonist. Nalbuphine is an analgesic (narcotic).
Uses
Analgesic;Opioid ligand
Manufacturing Process
To a slurry of 110.5 g of 14-hydroxydihydronormorphinone in 2.5 liters of
methylene chloride and 280 ml of triethylamine was added a solution of 106 g
of cyclobutanecarboxylic acid chloride in 500 ml of methylene chloride. The
temperature of the reaction mixture was maintained at 20°C to 25°C during
the addition. After 5 minutes the reaction mixture was brought to reflux and
heated for 5 hours.
It was then cooled, washed with water, dried over sodium sulfate and
evaporated to dryness. The residue was crystallized from benzene and
pentane to give 138.5 g of the dicyclobutanecarbonyl derivative, melting point
about 112°C (dec.).
The dicyclobutanecarbonyl derivative (136.7 g) was dissolved in 200 ml of
tetrahydrofuran and added dropwise to a suspension of 34.2 g of lithium
aluminum hydride in 1 liters of tetrahydrofuran. The temperature of the
mixture rose to reflux during the addition. Reflux was maintained for 2 hours
after the addition was completed. After cooling, 110 ml of ethyl acetate was
added dropwise, followed by 30 ml of water, followed by a solution of 53 g of
ammonium chloride in 125 ml of water. The resulting mixture was filtered and
the inorganic precipitate was washed with methanol. Evaporation of the
combined filtrates gave 66 g of N-cyclobutylmethyl-14-hydroxydihydronormorphinone, melting point 229°C to 231°C.
brand name
Nubain (Endo).
Therapeutic Function
Analgesic
Acquired resistance
Nalbuphine is an antagonist at μ receptors and an agonist at κ receptors. As an antagonist, it has approximately one-fourth the potency of naloxone, and it produces withdrawal when given to addicts. On a weight basis, the analgesic potency of nalbuphine approaches that of morphine. An intramuscular injection of 10 mg will give about the same degree and duration of analgesia as an equivalent dose of morphine.
Clinical Use
Nalbuphine is only available for parenteral dosage. Its elimination half-life is 2 to 3 hours. Metabolism of nalbuphine is by conjugation of the 3-OH group, and greater than 90% of the drug is excreted as conjugates in the feces.
Side effects
Side effects of nalbuphine are like those of other κ. Dysphoria is not as common as with pentazocine. Sedation is the most common side effect. Nalbuphine does not have the adverse cardiovascular properties found with pentazocine and butorphanol. Nalbuphine has low abuse potential and is not listed under the Controlled Substances Act.
NALBUPHINE HYDROCHLORIDE Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
23277-43-2(NALBUPHINE HYDROCHLORIDE)Related Search:
1of4