セルタコナゾール
セルタコナゾール 物理性質
- 融点 :
- 146-147°
- 沸点 :
- 614.1±55.0 °C(Predicted)
- 比重(密度) :
- 1.43±0.1 g/cm3(Predicted)
- 貯蔵温度 :
- Refrigerator
- 溶解性:
- DMSO(微量)、メタノール(微量)
- 外見 :
- 個体
- 酸解離定数(Pka):
- 6.68±0.12(Predicted)
- 色:
- 白色~淡黄色
- CAS データベース:
- 99592-32-2(CAS DataBase Reference)
安全性情報
- リスクと安全性に関する声明
- 危険有害性情報のコード(GHS)
セルタコナゾール 価格
| メーカー |
製品番号 |
製品説明 |
CAS番号 |
包装 |
価格 |
更新時間 |
購入 |
セルタコナゾール 化学特性,用途語,生産方法
効能
抗真菌薬
説明
Sertaconazole has been developed and launched for the
treatment of dermatological fungal infections by Ferrer
Internacional S. A. Mylan received FDA approval for
sertaconazole nitrate cream for the treatment of athlete's foot
(tinea pedis) at the end of 2003.
使用
An imidazole antifungal agent, inhibits the synthesis of ergosterol, an essential cell wall component of fungi.
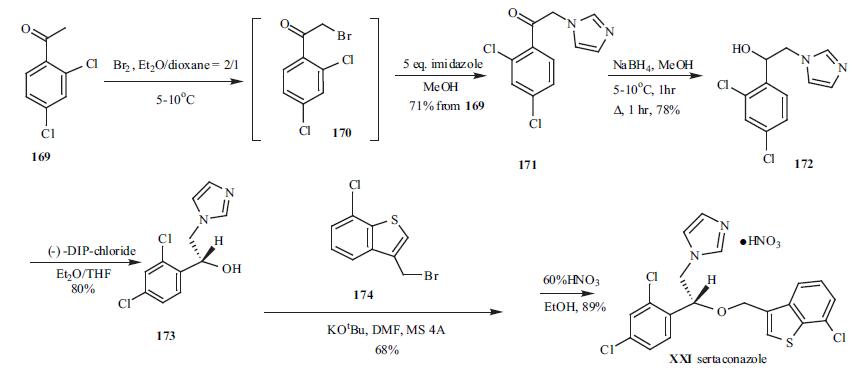
合成
2,4-Dichloro acetophenone
169 was brominated at low temperature to give bromide
intermediate 170, which was used without isolation. To the
same pot, five-fold excess of imidazole was added to give
imidazolylacetophenone 171 in 71% yield from 169.
Sodium borohydride was employed to reduce ketone 171 to
alcohol 172 in 78% yield. Racemic alcohol 172 was resolved with (-)-DIP-chloride to give its corresponding
chiral R-alcohol 173 in 80% yield. Compound 173 was then
alkylated with 3-bromomethyl-7-chlorobenzo[b]thiophene
(174) in dry DMF in the presence of potassium t-butoxide to
give the alkylation product in 68% yield. Finally, 60%
nitric acid was used to make sertaconazole mononitrate
(XXI) in 89% yield.
Solubility in organics
Fairly soluble in ethanol (1.7 %), chloroform (1.5 %); slightly soluble in acetone (0.95 %); very slightly soluble in noctanol (0.069 %). Practically insoluble in water (< 0.01 %).
セルタコナゾール 上流と下流の製品情報
原材料
準備製品
セルタコナゾール 生産企業
Global( 134)Suppliers
99592-32-2(セルタコナゾール)キーワード:
- 99592-32-2
- 1-(2-((7-chlorobenzo(b)thien-3-yl)methoxy)-2-(2,4-dichlorophenyl)ethyl)-1h-i
- Dermofix
- FI-7056
- Zalain
- 1-[2-[(7-Chlorobenzo[b]thiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole
- 1-[β-[(7-Chlorobenzo[b]thiophene-3-yl)methoxy]-2,4-dichlorophenethyl]-1H-imidazole
- Sertaconazol
- (+-)-1-(2,4-Dichloro-beta-((7-chlorobenzo(b)thien-3-yl)methoxy)phenethyl)imidazole
- 1h-imidazole,1-(2-((7-chlorobenzo(b)thien-3-yl)methoxy)-2-(2,4-dichlorophenyl)
- 7-chloro-3-(1-(2,4-dichlorophenyl)-2-(1h-imidazol-1-yl)ethoxy-methyl)benzo(b
- 7-cloro-3-(1-(2,4-diclorofenil)-2-(1h-imidazol-1-il)etoxi-metil)benzo(b)tiof
- fi-7045
- 7-CHLORO-3-[1-(2,4-DICHLOROPHENYL)-2-(1H-IMIDAZOL-1-YL)ETHOXY-METHYL] BENZO[B]THIOPHENE NITRATE
- 1-[2-[(7-chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]imidazole nitrate
- 1-[2-[(7-Chlorobenzo[b]thien-3-y1)methoxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole
- Sertaconazole nitrate
- 1H-Imidazole, 1-[2-[(7-chlorobenzo[b]thien-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-
- Sertaconazole nitrate USP/EP/BP
- Sertaconazole API
- eno
- SERTACONAZOLE
- (RS)-1-(2-((7-chloro-1-benzothiophen-3-yl)methoxy)-2-(2,4-dichlorophenyl)ethyl) -1H-imidazole
- 1-[2-[(7-クロロベンゾ[b]チオフェン-3-イル)メトキシ]-2-(2,4-ジクロロフェニル)エチル]-1H-イミダゾール
- セルタコナゾール
- 7-クロロ-3-[[1-(2,4-ジクロロフェニル)-2-(1H-イミダゾール-1-イル)エトキシ]メチル]-1-ベンゾチオフェン
- 1-[β-[(7-クロロベンゾ[b]チオフェン-3-イル)メトキシ]-2,4-ジクロロフェネチル]-1H-イミダゾール
- 1-{2-[(7-クロロ-1-ベンゾチオフェン-3-イル)メトキシ]-2-(2,4-ジクロロフェニル)エチル}-1H-イミダゾール