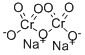
중크롬산나트륨 C화학적 특성, 용도, 생산
화학적 성질
Sodium chromate, including the hexahydrate, is yellow crystalline solids that can also be used in solution. Disodium dichromate (10588-01-9):
용도
Sodium dichromate is red solid, soluble, powerful oxidizing agent, and consequently a fire hazard with dry carbonaceous materials. Formed by acidifying sodium chromate solution, and then evaporating. Used (1) in matches and pyrotechnics, (2) in leather tanning and in the textile industry, (3) as a source of chromate, cheaper than potassium dichromate.
일반 설명
A red or red-orange crystalline solid. May be strongly irritating to skin, eyes and mucous membranes. Used as a corrosion inhibitor, and in the manufacture of other chemicals.
공기와 물의 반응
Deliquescent. Soluble in water.
반응 프로필
SODIUM DICHROMATE is a strong oxidizing agent. Incompatible with strong acids. . Contact with combustible materials may lead to fires. Toxic chromium oxide fumes may form in fire [USCG, 1999]. The well known "chromic acid mixture" of dichromate and sulfuric acid with organic residue led to violent exothermic reaction. This mixture in combination with acetone residue also led to violent reaction. The combination of the dichromate and sulfuric acid with alcohols, ethanol and 2-propanol, led to violent exothermic reaction. Because of the occurrence of many incidents involving the dichromate-sulfuric acid mix with oxidizable organic materials, SODIUM DICHROMATE is probably best to avoid such interactions. The combination of the dichromate with hydrazine is explosive (one may expect the reaction of the dichromate to be vigorous with amines in general), [Mellor, 1943, Vol. 11, 234]. The addition of the dehydrated dichromate salt to acetic anhydride led to an exothermic reaction which eventually exploded. An induction period proceeded the explosion event [Bretherick, 5th Ed., 1995]. Boron, silicon, and dichromates form pyrotechnic mixtures. A mixture of acetic acid, 2-methyl-2-pentenal and the dichromate led to a runaway reaction and eruption of the reactor contents, [J. Haz. Mat., 1987, 233-239].
건강위험
Inhalation of dust or mist causes respiratory irritation sometimes resembling asthma; nasal septal perforation may occur. Ingestion causes vomiting, diarrhea, and (rarely) stomach and kidney complications. Contact with eyes or skin produces local irritation; repeated skin exposure causes dermatitis.
화재위험
Behavior in Fire: Decomposes to produce oxygen when heated. May ignite other combustibles upon contact.
Safety Profile
Confirmed carcinogen with experimental tumorigenic data. Poison by ingestion, sktn contact, intravenous, intraperitoneal, and subcutaneous routes. Human systemic effects by ingestion: cough, nausea or vomiting, and sweating. Human mutation data reported. A caustic and irritant. A powerful oxidizer. Potentially explosive reaction with acetic anhydride, ethanol + sulfuric acid + heat, hydrazine. Violent reaction or ignition with boron + shcon (pyrotechnic), organic residues + sulfuric acid, 2-propanol + sulfuric acid, sulfuric acid + trinitrotoluene. Incompatible with hydroxylamine. When heated to decomposition it emits toxic fumes of Na2O. See also CHROMIUM COMPOUNDS.
잠재적 노출
Used to make dyes, inks, pigments, and other chromates; in leather tanning, a corrosion inhibitor in circulating water systems; metal treatment; a drilling mud additive; chemical intermediate for chromium catalysts; colorimetry, oxidizing agent; bleaching agent; an algicide, fungicide, insecticide; in wood preservation.
운송 방법
UN3087 Oxidizing solid, toxic, n.o.s., Hazard Class: 5.1; Labels: 5.1-Oxidizer, 6.1-Poisonous materials, Technical Name Required. UN3085 Oxidizing solid, corrosive, n.o.s., Hazard Class: 5.1; Labels: 5.1-Oxidizer, 8-Corrosive material, Technical Name Required.
비 호환성
Aqueous solution in a base. A strong oxidizer. Violent reaction with reducing agents; combustibles, strong acids; organic materials.
중크롬산나트륨 준비 용품 및 원자재
원자재
준비 용품