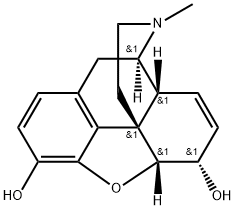
모르핀
|
|
모르핀 속성
- 녹는점
- 255°C
- 끓는 점
- 427.77°C (rough estimate)
- 밀도
- 1.0864 (rough estimate)
- 굴절률
- 1.5400 (estimate)
- 인화점
- 11 °C
- 저장 조건
- −20°C
- 용해도
- 에탄올(약간), 메탄올(약간)에 용해됨
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 산도 계수 (pKa)
- 8.21(at 25℃)
- 색상
- 흰색에서 연한 노란색
- 수용성
- 0.4mg/L(25℃)
- BCS Class
- 1,3
- CAS 데이터베이스
- 57-27-2
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-23/24/25-39/23/24/25 | ||
| 안전지침서 | 7-16-36/37-45 | ||
| 유엔번호(UN No.) | UN 1230 3/PG 2 | ||
| WGK 독일 | 2 | ||
| 유해 물질 데이터 | 57-27-2(Hazardous Substances Data) | ||
| 독성 | An alkaloid of the opium poppy that makes up between 9% and 14% of good grades of opium. Morphine is usually used clinically as an analgesic in the form of the sulfate or hydrochloride salt. The most important acute toxic effect of large doses of morphine is depression of the respiratory centers in the medulla and pons. Morphine and related drugs also cause somnolence, coma, cold clammy skin, bradycardia, and hypotension. Initial doses of morphine seem to stimulate the chemoreceptor trigger zone to induce emesis, with subsequent doses blocking the vomiting center, hence blocking emesis. Morphine also has profound effects on the gastrointestinal tract, increasing the tone of the intestinal tract, but decreasing the propulsive or spasmodic reflexes, thus resulting in constipation. Morphine stimulates the nucleus of the third cranial nerve to produce miosis, making pinpoint pupil a diagnostic sign both in morphine overdose and morphine addiction. Morphine causes a variety of effects on the CNS and is highly addictive. Many behavioral changes are seen, ranging from euphoria to sedation. These behavioral effects contribute to the problem of abuse with all of the opiates. Tolerance and dependence occur with repeated dosing, with increasingly larger doses being needed to obtain the original effect. Abrupt withdrawal after chronic use can lead to physiological rebound in these same systems. Therapy for acute overdosage involves physiological support (establishment of adequate respiratory exchange), gastric lavage, and use of narcotic antagonists. Morphine and related compounds act by binding to specific high-affinity receptors concentrated in the nervous system, but also located elsewhere in the body. In the nervous system, the endogenous ligands for these morphine receptors are the opioid peptides that include the enkephalins, endorphins, and dynorphins. The multiple and complex actions of morphine are due, in part, to the fact that it acts as an agonist at many of these classes of receptors. Paregoric (camphorated tincture of opium) is used as an antidiarrheal. Paregoric is a schedule III drug under the US Controlled Substances Act and may produce physical dependence. |
모르핀 C화학적 특성, 용도, 생산
개요
Morphine is the principal alkaloid obtained from opium. Opium is the resinous latex that exudes from the seed pod of the opium poppy, Papver somneferum, when it is lacerated. Alkaloids account for approximately 25% of opium, and of this 25% about 60% is morphine.화학적 성질
White, crystalline alkaloid. Slightly soluble in water,alcohol, and ether.물리적 성질
Appearance: white or almost white crystalline powder or colorless, silky needles or cubical masses, efflorescent in a dry atmosphere. Solubility: soluble in water, slightly soluble in ethanol, practically insoluble in chloroform or ether. Specific optical rotation: ?110.0° to 115.0°역사
Morphine has a strong analgesic effect, especially for moderate and severe cancer pain. However, morphine has serious side effects, such as addiction, respiratory depression, acute poisoning, and death. Therefore, in regulating the clinical application of morphine at the same time, it is necessary to explore the alternative to morphine with strong analgesic role and minor side effects.In the 1970s, tramadol was launched and marketed as “Tramal” by the German pharmaceutical company Grünenthal GmbH in West Germany , and 20 years later, it was launched in countries such as the United Kingdom, the United States, and Australia. It is marketed in many brand names worldwide. After R&D of more than 100 years, opioid drugs were increased and have been widely used in clinic as analgesic drugs.
용도
A degradation product of Morphine. A dimolecular base formed by the gentle oxidation of Morphine in alkaline solution. Name Pseudomorphine is also used for the C17 alkaloid base정의
ChEBI: A morphinane alkaloid that is a highly potent opiate analgesic psychoactive drug. Morphine acts directly on the central nervous system (CNS) to relieve pain but has a high potential for addiction, with tolerance and both physical and psychological dependen e developing rapidly. Morphine is the most abundant opiate found in Papaver somniferum (the opium poppy).생산 방법
Morphine is detoxified or biotransformed mainly in the liver by conjugation with glucuronic acid. Morphine is conjugated by a series of reactions involving the formation of uridine diphosphoglucose (UDP-glucose), the oxidation of carbon-6 of glucose to form uridine diphosphoglucuronic acid (UDP-glucuronic acid) and the transfer of glucuronic acid to morphine to form the morphine glucuronide. The following enzymes catalyze the sequential reactions; reaction(1), UDP-glucose pyrophosphorylase; reaction (2), UDP-glucose dehydrogenase; reaction (3), glucuronyl transferase; reaction (4) nucleoside diphosphokinase.Indications
Morphine is mainly used to treat both acute and chronic severe pain. It is also used for pain caused by myocardial infarction and for labor pains. Morphine relieves pulmonary edema symptoms; anesthesia and preoperative administration can make the patient quiet and drowsy; compound formula of morphine was used for acute and chronic diarrhea.원료
It is important to remember that a minor change in the structure of morphine (or any other opioid) will likely cause a different change in the affinity and intrinsic activity of the new compound at each of the opioid receptor types. Thus, the opioid receptor selectivity profile of the new compound may be different than the structure from which it was made or modeled (i.e., a selective μ agonist may shift to become a selective κ agonist, etc.). In addition, the new compound will have different physicochemical properties than its parent. The different physicochemical properties (e.g., solubility, partition coefficient, and pKa) will result in different pharmacokinetic characteristics for the new drug and can affect its in vivo activity profile. For example, a new drug (Drug A) that is more lipophilic than its parent may distribute better to the brain and appear to be more active, whereas in actuality, it may have lower affinity or intrinsic activity for the receptor. The greater concentration of Drug A reaching the brain is able to overcome its decreased agonist effect at the receptor.위험도
Narcotic, habit-forming drug, salerestricted by law in the U.S.색상 색인 번호
Morphine bitartrate caused contact dermatitis in a worker at a plant producing opium alkaloids. Morphine hydrochloride and morphine bitartrate showed patchtest- positive reactions in another patient with contact dermatitis working in the production of concentrated poppy straw. We observed a concomitant reaction between a morphine base and a codeine base in a patient with drug skin eruption due to codeine.Pharmacokinetics
Morphine is the prototype opioid. It is selective for μ opioid receptors. The structure of morphine is composed of five fused rings, and the molecule has five chiral centers with absolute stereochemistry 5(R), 6(S), 9(R), 13(S) and 14(R). The naturally occurring isomer of morphine is levo-[(–)] rotatory. (+)-Morphine has been synthesized, and it is devoid of analgesic and other opioid activities.Safety Profile
Poison experimentally by ingestion, intracerebral, intraperitoneal, subcutaneous, and intravenous routes. Human reproductive effects by an unspecified route: effects on newborn, including drug dependence. Experimental reproductive effects. Mutation data reported.Morphine is the constituent of opium most responsible for its toxic effects. When taken orally, the effects of morphine poisoning begin to appear in 20-40 minutes; if taken hypodermically, the symptoms appear much earlier and narcotism is more likely to follow the early symptoms. Abuse leads to habituation or adlction. Inlvidual susceptibility varies greatly and children are more susceptible than adults. When heated to decomposition it emits toxic fumes of NOx.
Purification Methods
Crystallise the narcotic from MeOH or anisole. It dehydrates at 130o. Its solubility in H2O is 0.2g/L at 20o and 0.9g/L at 100o, and in EtOH it is 5g/L at 20o and 10g/L on boiling. The styphnate has m 189o (from aqueous EtOH). [Beilstein 27 II 118, 27 III/IV 2223.]참고 문헌
Derosne., Ann. Chirn., 45, 257 (1803)Laurent., Ann. Chirn. Phys., 19, iii, 359 (1847)
Muller., Apoth. Zeit., 18, 257 (1903)
Mannich., Chern. Zentr., II, 820 (1916)
Emde., Helv. Chirn. Acta, 13, 1035 (1930)
Gates, Tschudi., 1. Arner. Chern. Soc., 78, 1380 (1956)
Crystal structure: MacKay, Hodgkin., 1. Chern. Soc., 3261 (1955)
Synthesis: Gates, TschudL,J. Arner. Chern. Soc., 74, 1109 (1952)
Elad, Ginsburg., ibid, 76,312 (1954)
Elad, Ginsburg., J. Chern. Soc., 3052 (1954)
Morrison, Waite, Shavel., Tetrahedron Lett., 4055 (1967)
NMR and mass spectra: Rull., Bull. Soc. Chim. Fr., 586 (1963)
Okuda et al., Chern. Pharrn. Bull. (Tokyo), 11, 1465 (1963)
Wheeler, Kinstle, Rinehart., J. Arner. Chern. Soc., 89,4494 (1967)
Biosynthesis: Barton et aI., J. Chern. Soc., 2423 (1965)
Pharmacology: Vahlen., Arch. expo Path. Pharrn., 47, 368 (1902)
Small et al., Public Health Reports, Suppl. 138, Washington (1938)
Krueger, Eddy, Sumwalt., ibid, No. 165, Washington (1943)
모르핀 준비 용품 및 원자재
원자재
준비 용품
모르핀 관련 검색:
헤로인
3-METHYLMORPHINE SULFATE SALT
MORPHINIUM CHLORIDE
MORPHIN-6-GLUCURONID
MORPHINE N-OXIDE
7,8-DIDEHYDRO-4,5-EPOXY-17-[METHYL-D3]MORPHINAN-3,6-DIOL HYDROCHLORIDE: TRIHYDRATE
MORPHINE
CODEINE PHOSPHATE
CODEINE HYDROCHLORIDE
CODEINE
Morphine Sulfate
ETHYLMORPHINE
OPIUM
6-ACETYLCODEINE
Pseudomorphine (Morphine Impurity)
6-ACETYLMORPHINE
CODEINE N-OXIDE
DIACETYLMORPHINE HYDROCHLORIDE CI (25 MG) (AS) (HEROIN HYDROCHLORIDE)