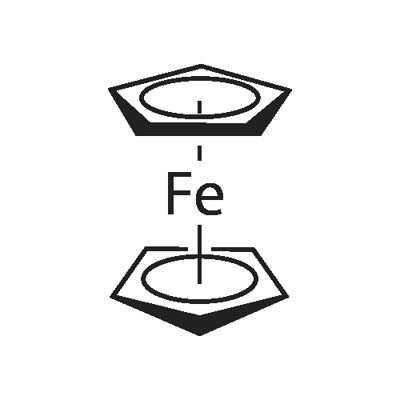
디시클로펜타디에닐 철
|
|
디시클로펜타디에닐 철 속성
- 녹는점
- 172-174 °C (lit.)
- 끓는 점
- 249 °C (lit.)
- 밀도
- 1.490
- 증기압
- 0.03 mm Hg ( 40 °C)
- 인화점
- 100°C
- 저장 조건
- Store below +30°C.
- 용해도
- insoluble in H2O; soluble in ethanol, ethyl ether,benzene, dilute HNO 3
- 물리적 상태
- 수정 같은
- 색상
- 오렌지 레드
- 색상
- 오렌지색
- 수용성
- 실질적으로 불용성
- 감도
- Air & Moisture Sensitive
- 승화점
- 100 ºC
- Merck
- 14,4037
- 노출 한도
- ACGIH: TWA 10 mg/m3; TWA 1 mg/m3
OSHA: TWA 15 mg/m3; TWA 5 mg/m3
NIOSH: TWA 10 mg/m3; TWA 5 mg/m3; TWA 1 mg/m3
- 안정성
- 실온에서 안정함. 강한 산화제와 호환되지 않습니다. 가연성이 높습니다.
- LogP
- 3.711 at 22℃
- CAS 데이터베이스
- 102-54-5(CAS DataBase Reference)
- NIST
- Ferrocene(102-54-5)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,Xn,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-22-51/53-2017/11/22 | ||
| 안전지침서 | 61-22-24/25 | ||
| 유엔번호(UN No.) | UN 1325 4.1/PG 2 | ||
| OEB | B | ||
| OEL | TWA: 10 mg/m3 (total) | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | LK0700000 | ||
| 자연 발화 온도 | >150 °C | ||
| TSCA | Yes | ||
| 위험 등급 | 4.1 | ||
| 포장분류 | II | ||
| HS 번호 | 29310095 | ||
| 유해 물질 데이터 | 102-54-5(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 1320 mg/kg LD50 dermal Rat > 3000 mg/kg | ||
| 기존화학 물질 | 99-3-1231 |
디시클로펜타디에닐 철 C화학적 특성, 용도, 생산
화학적 성질
Ferrocene, a metallocene, is a bright orange salt-like crystals from alcohol. Camphor odor.물리적 성질
Orange crystals; camphor-like odor; melts at 172.5°C; vaporizes at 249°C; sublimes above 100°C; thermally stable above 500°C; insoluble in water; soluble in alcohol, ether and benzene; also soluble in dilute nitric acid and concentrated sulfuric acid forming a deep red solution that fluoresces.용도
Ferrocene is used as a catalyst for vulcanization, acceleration, and polymerization, as a chemical intermediate for polymeric compounds such as high temperature polymers, as an antiknock additive for gasoline, as a coating for missiles and satellites, and as a high-temperature lubricant.생산 방법
Ferrocene is produced from the reaction of cyclopentadiene with reduced iron in the presence of metal oxides. There is also a two-stage production process in which produced iron (II)oxide (stage 1) is reacted with cyclopentadiene.정의
ferrocene: An orange-red crystallinesolid, Fe(C5H5)2; m.p. 173°C. Itcan be made by adding the ioniccompound Na+C5H5- (cyclopentadienylsodium, made from sodium andcyclopentadiene) to iron(III) chloride.In ferrocene, the two rings are parallel,with the iron ion sandwiched betweenthem (hence the namesandwich compound: see formula).The bonding is between pi orbitalson the rings and d-orbitals on theFe2+ ion. The compound can undergoelectrophilic substitution on theC5H5rings (they have some aromatic character).It can also be oxidized to theblue ion (C5H5)2Fe+. Ferrocene is the first of a class of similar complexescalled sandwich compounds. Its systematicname is di-π-cyclopentadienyliron(II).제조 방법
Dicyclopentadienyliron may be obtained in a single-step synthetic route by heating cyclopentadiene with iron or iron pentacarbonyl at 300°C:2C5H5 + Fe → (C5H5)2Fe
Also, it can be prepared by the reaction of iron(II) chloride with cyclopentadiene in the presence of an alkyl amine or a similar base.
Another convenient method of preparing this π-complex of iron is a twostep process in which the first step involves preparation of cyclopentadienyl Grignard reagent, such as 2,4-cyclopentadienylmagnesium bromide C5H5MgBr which may then be combined with ferric chloride to yield dicyclopentadienyl iron:
3C5H5MgBr + FeCl3 → (C5H5)2Fe + 3MgBrCl
Another general method of preparation involves the reaction of cyclopentadiene with sodium metal or sodium hydride in tetrahydrofuran (THF). Addition of iron(II) chloride to this solution forms the complex dicyclopentadienyliron:
2C5H6 + 2Na → 2C5H5ˉ + 2Na+ + H2
In 3:2 molar ratio of cyclopentadiene to sodium cyclopentene is obtained along with cyclopentadienidide (C5H5ˉ ) anion:
3C5H6 + 2Na → 2C5H5¯ + 2Na+ + C5H8
FeCl2 + 2C5H6Na → (C5H5)2Fe + 2NaCl
일반 설명
Orange crystalline solid or orange-yellow powder. Sublimes above 212°F. Camphor odor.공기와 물의 반응
Sensitive to prolonged exposure to air and may be sensitive to light. Insoluble in water.반응 프로필
Ferrocene reacts violently with tetranitromethane. . Contact of tetranitromethane with Ferrocene under various conditions leads to violent explosion, [Trans. Met. Chem., 1979, 4, 207-208].위험도
Moderate fire risk. Evolves toxic products on decomposition and heating.건강위험
Dicyclopentadienyl iron causes changes in blood parameters and hepatic cirrhosis.The toxicological properties of dicyclopentadienyl iron have not been extensively investigated. However, it has been used as a preventive and therapeutic iron deficiency drug, and its utilization is listed as tolerable.
화재위험
Flash point data for Ferrocene are not available. Ferrocene is probably combustible.Safety Profile
Poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Flammable; reacts violently with NH4ClO4. When heated to decomposition it emits acrid smoke and irritating fumes.잠재적 노출
Used as additive in fuel oil; antiknock agent in gasoline fuel; used in making rubber, silicone resins, high-temperature polymers and lubricants; interme diate for high-temperature polymers; as a smoke suppres sant and catalystCarcinogenicity
Ferrocene was administered by intramuscular injection at a dose of 5175 mg/kg/2 years. By the criterion established by the Registry of Toxic Effects of Chemical Substances (RTECS), ferrocene was an equivocal tumorigenic agent and tumors were most evident at the site of multiple injections.운송 방법
UN1325 Flammable solids, organic, n.o.s., Hazard Class: 4.1; Labels: 4.1-Flammable solid.Purification Methods
Purify it by crystallisation from pentane or cyclohexane (also *C6H6 or MeOH can be used). It is moderately soluble in Et2O and sublimes readily above 100o. Crystallisation from EtOH gave material m 172.5-173o. [Wilkinson Org Synth Coll Vol IV 473 1963, Miller J Chem Soc 632 1952.] It has also been crystallised from methanol and sublimed in vacuo. [Saltiel et al. J Am Chem Soc 109 1209 1987, Beilstein 16 IV 1783.]비 호환성
Violent reaction with ammonium per chlorate, tetranitromethane, mercury(II) nitrate. Incompa tible with oxidizers (chlorates, nitrates, peroxides, perman ganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.Peroxomonosulfuric acid. Decomposes @≧465 ℃.
디시클로펜타디에닐 철 준비 용품 및 원자재
원자재
준비 용품
1,1'-비스(디-t-부틸포스피노)페로센팔라듐디클로라이드,
1,1′-페로센다이카르복스알데하이드
1 1'-BIS(DIPHENYLPHOSPHINO)FERROCENE
1,1'- 비스 (디 페닐 포스 피노) 페로센
1,1'-DI-N-부틸페로센
1,1'-Bis(di-tert-butylphosphino)ferrocene
Ferrocene derivative
dibuty cebacate with ferric chloride
뷰틸페로센
1,1'-DIMETHYLFERROCENE
1,1'-디아세틸페로센
1-DIPHENYLPHOSPHINO-1'-(DI-TERT-BUTYLPH&
1,1'-BIS(DIISOPROPYLPHOSPHINO)페로센
Chlorocarbonyl ferrocene
1,1'-디에틸페로센
2-메틸시클로펜타디에닐 망간트리카르보닐
벤조일페로센
FERROCENEBORONIC ACID
1,1'-binaphthalene-2,2'-diyl diacetate
Acetylferrocene
디시클로펜타디에닐 철 공급 업체
글로벌( 550)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| PANJIN BAFANG INDUSTIAL CO.,LTD | +undefined18525718838 |
xinaobf888@163.com | China | 1 | 58 |
| Changyi Longchang Bio-Chemical Co., LTD | +86-13256193735; +8613256193735 |
sale01@longchangchemical.com | China | 990 | 58 |
| Dalian Richfortune Chemicals Co., Ltd | 0411-84820922 8613904096939 |
sales@richfortunechem.com | China | 304 | 57 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17367 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5873 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 |
sales02@sdzhonghuimaterial.com | China | 983 | 58 |
| Shijiazhuang Jianxin Biotechnology Co., LTD | +undefined-86-1906231316-9 +undefined86-19062313169 |
liyingjian1997@gmail.com | China | 709 | 58 |
| Xiamen Eagle Chemical Limited Corporation | +86-5925023701 +86-18900207489 |
yiyunchem@163.com | China | 6043 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615350571055 |
Sibel@weibangbio.com | China | 5848 | 58 |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-17333973358 |
sales06@hbduling.cn | China | 8056 | 58 |
디시클로펜타디에닐 철 관련 검색:
사이클로펜타디에닐(포밀사이클로펜타디에닐)철 엑소-테트라히드로디사이클로펜타디엔 디사이클로펜타디엔 페로센메탄올 시클로펜타디엔 페로센 카르복실 산 (S)-(+)-3-하이드록시 사하이드로 퓨란 1,1'- 비스 (디 페닐 포스 피노) 페로센 (디메틸아미노메틸)페로센 디클로로 [1,1'- 비스 (디 페닐 포스 피노) 페로센] 팔라듐 (II) 1,1′-페로센다이카르복실 산 디시클로펜타디에닐 철
BIS(CYCLOPENTADIENYL)MANGANESE
beryllium dicyclopenta-2,4-dienide
Chromocene
Ferrocene, acetyl-
polydicyclopentadiene
3-Hydroxytetrahydrofuran