펜탄
|
|
펜탄 속성
- 녹는점
- -130 °C
- 끓는 점
- 36 °C
- 밀도
- 0.626 g/mL at 25 °C(lit.)
- 증기 밀도
- 2.48 (vs air)
- 증기압
- 26.98 psi ( 55 °C)
- 굴절률
- n
20/D 1.358
- 인화점
- −57 °F
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- 에탄올: 용해성(lit.)
- 물리적 상태
- 액체
- 산도 계수 (pKa)
- >14 (Schwarzenbach et al., 1993)
- 색상
- 무색의
- Specific Gravity
- 0.63
- 상대극성
- 0.009
- 냄새
- 휘발유처럼.
- Odor Threshold
- 1.4ppm
- 폭발한계
- 1.4-8%(V)
- 수용성
- 불용성
- 최대 파장(λmax)
- λ: 200 nm Amax: ≤0.70
λ: 210 nm Amax: ≤0.20
λ: 220 nm Amax: ≤0.07
λ: 230 nm Amax: ≤0.02
λ: 250 nm Amax: ≤0.004
- Merck
- 14,7116
- BRN
- 969132
- Henry's Law Constant
- 1.20 at 25 °C (J?nsson et al., 1982)
- 노출 한도
- TLV-TWA 600 ppm (~1800 mg/m3) (ACGIH), 1000 ppm (~3000 mg/m3) (OSHA), 500 ppm (~1500 mg/m3) (MSHA); STEL 750 ppm (~2250 mg/m3) (ACGIH).
- Dielectric constant
- 1.8(20℃)
- InChIKey
- OFBQJSOFQDEBGM-UHFFFAOYSA-N
- LogP
- 3.390
- CAS 데이터베이스
- 109-66-0(CAS DataBase Reference)
- NIST
- Pentane(109-66-0)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F+,Xn,N,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 12-51/53-65-66-67 | ||
| 안전지침서 | 9-16-29-33-61-62 | ||
| 유엔번호(UN No.) | UN 1265 3/PG 2 | ||
| OEB | A | ||
| OEL | TWA: 120 ppm (350 mg/m3), Ceiling: 610 ppm (1800 mg/m3) [15-minute] | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | RZ9450000 | ||
| F 고인화성물질 | 3-10 | ||
| 자연 발화 온도 | 500 °F | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | II | ||
| HS 번호 | 29011090 | ||
| 유해 물질 데이터 | 109-66-0(Hazardous Substances Data) | ||
| 독성 | LC (in air) in mice: 377 mg/l (Fühner) | ||
| IDLA | 1,500 ppm [10% LEL] | ||
| 기존화학 물질 | KE-27968 |
펜탄 C화학적 특성, 용도, 생산
개요
펜테인은 펜탄이라고도 불리는 탄소와 수소의 결합으로 이루어진 알케인 탄화수소로,상온에서 상쾌한 냄새가 나는 무색의 투명한 액체 상태를 유지하며.용도
펜탄은 주로 EPS, PIR 등과 같은 폴리스티렌 폼의 제조에서 주요 발포제로 사용됩니다.이 제품은 다른 화학 물질에 비해 비용이 저렴하고 사용시 낮은 끓는점 및 상대적 안전성 때문에 사용됩니다.용도
일반 펜탄은 또한 용제 및 제약, 석유 화학, 도료 및 코팅과 같은 다양한 산업 분야에서 사용됩니다.펜탄은 또한 실험실에서 용제로 사용됩니다.안전성
극도의 인화성, 공기와의 폭발성 혼합물을 형성하며, 열원 또는 화염에 노출되면 연소 및 폭발 할 수 있습니다. 가열 된 용기에서 폭발 할 수도 있음. 산화제와 격렬하게 반응 할 것. 그것은 공기보다 무겁고 물에 녹지 않으며, 낮은 수준의 먼 지역으로 퍼지고, 점화원이 될 경우 파업을합니다.화학적 성질
n-Pentane is a flammable liquid. It has applications in industry as an aerosol propellant and as an important component of engine fuel.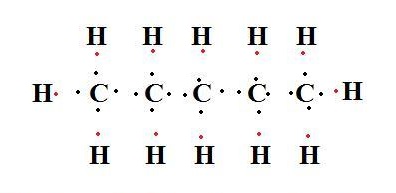
pentane lewis structure
N-propane is a CNS depressant. Studies with dogs have indicated that it induces cardiac sensitization. In high concentrations it causes incoordination and inhibition of the righting refl exes.
N-pentane is being used primarily in Europe in integral-skin flexible foams. Pentane is twice as effective as CFC-11 as a blowing agent.
물리적 성질
Clear, colorless, volatile liquid with an odor resembling gasoline. An odor threshold concentration of 1.4 ppmv was reported by Nagata and Takeuchi (1990).용도
n-Pentane occurs in volatile petroleum fractions(gasoline) and as a constituent ofpetroleum ether. It is used as a solvent, in themanufacture of low-temperature thermometers,and as a blowing agent for plastics.정의
pentane: A straight-chain alkanehydrocarbon, C5H12; r.d. 0.63; m.p.–129.7°C; b.p. 36.1°C. It is obtainedby distillation of petroleum.생산 방법
Pentane is produced by fractional distillation of natural gas liquids and crude oil. It is also produced by the catalytic crackdown of naphtha.일반 설명
A clear colorless liquid with a petroleum-like odor. Flash point 57°F. Boiling point 97°F. Less dense than water and insoluble in water. Hence floats on water. Vapors are heavier than air.공기와 물의 반응
Highly flammable. Insoluble in water.반응 프로필
Pentane is incompatible with strong oxidizers. Pentane is also incompatible with strong acids, alkalis, and oxygen. Mixtures with chlorine gas have produced explosions. Pentane will attack some forms of plastics, rubber, and coatings. .건강위험
n-Pentane did not exhibit any marked toxicityin animals. However, inhalation ofits vapors at high concentrations can causenarcosis and irritation of the respiratorypassages. Such effects may be observedwithin the range 5–10% concentration inair. In humans, inhalation of 5000 ppm for10 minutes did not cause respiratory tractirritation or other symptoms (Patty and Yant1929).There is no report in the literature indicatingany adverse effects from pentaneother than narcosis and irritation. An intravenousLD50 value in mouse is recorded as446 mg/kg (NIOSH 1986).
화재위험
Behavior in Fire: Containers may explode화학 반응
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Safety Profile
Moderately toxic by inhalation and intravenous routes. Narcotic in high concentration. The liquid can cause blisters on contact. Flammable liquid. Highly dangerous fire hazard when exposed to heat, flame, or oxidizers. Severe explosion hazard when exposed to heat or flame. Shock can shatter metal containers and release contents. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes잠재적 노출
Pentane is used in manufacture of ice, low-temperature thermometers; in solvent extraction processes; as a blowing agent in plastics; as a fuel; as a chemical intermediate (for amylchlorides, e.g.).환경귀착
Biological. n-Pentane may biodegrade in two ways. The first is the formation of pentyl hydroperoxide, which decomposes to 1-pentanol followed by oxidation to pentanoic acid. The other pathway involves dehydrogenation to 1-pentene, which may react with water giving 1- pentanol (Dugan, 1972). Microorganisms can oxidize alkanes under aerobic conditions (Singer and Finnerty, 1984). The most common degradative pathway involves the oxidation of the terminal methyl group forming 1-pentanol. The alcohol may undergo a series of dehydrogenation steps forming an aldehyde (valeraldehyde) then a fatty acid (valeric acid). The fatty acid may then be metabolized by β-oxidation to form the mineralization products, carbon dioxide and water (Singer and Finnerty, 1984). Mycobacterium smegnatis was capable of degrading pentane to 2- pentanone (Riser-Roberts, 1992).Photolytic. When synthetic air containing gaseous nitrous acid and pentane was exposed to artificial sunlight (λ = 300–450 nm) methyl nitrate, pentyl nitrate, peroxyacetal nitrate, and peroxypropionyl nitrate formed as products (Cox et al., 1980).
Chemical/Physical. Complete combustion in air yields carbon dioxide and water. Pentane will not hydrolyze because it does not contain a hydrolyzable functional group.
운송 방법
UN1265 Pentanes Hazard Class: 3; Labels: 3-Flammable liquid.Purification Methods
Stir the pentane with successive portions of conc H2SO4 until there is no further coloration during 12hours, then with 0.5N KMnO4 in 3M H2SO4 for 12hours, wash with water and aqueous NaHCO3. Dry it with MgSO4 or Na2SO4, then P2O5 and fractionally distil it through a column packed with glass helices. It is also purified by passage through a column of silica gel, followed by distillation and storage with sodium hydride. An alternative purification is by azeotropic distillation with MeOH, which is subsequently washed out from the distillate (using water), followed by drying and re-distilling. For removal of carbonyl-containing impurities, see n-heptane. Also purify it by fractional freezing (ca 40%) on a copper coil through which cold air is passed, then wash with conc H2SO4 and fractionally distil it. [Beilstein 1 IV 303.]비 호환성
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Attacks some plastics, rubbers, and coatings.폐기물 처리
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.펜탄 준비 용품 및 원자재
원자재
준비 용품
5-METHOXY-QUINAZOLIN-4-YLAMINE
n-헵탄올
2-Amino-5-nitropyrimidine
1,4-Dibromopentane
2-Bromo-5-hydroxypyridine radical ion(1+)
3-아미노피리딜염산염
EUROPIUM D-3-TRIFLUOROACETYLCAMPHORATE
Cefepime
테트라에틸렌 글리콜 부틸 에테르
디 -tert- 부틸 클로로 포스 핀
헵테노포스
1-Cyclopentenecarboxylic acid
ORNOPROSTIL
4-BROMO-3-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOLE
2,2,4,6,7-Pentamethyldihydrobenzofuran-5-sulfonyl chloride
3-TRIFLUOROACETYL-D-캠퍼
터트-뷰틸클로로다이메틸실란
1-AMINOCYCLOPROPANECARBONITRILE
Ketamine hydrochloride
Bis(pinacolato)diboron
4-Benzyloxybenzeneboronic acid
1-TERT-BUTYL-3-(TRIFLUOROMETHYL)-1H-PYRAZOLE-4-CARBOXYLIC ACID
4,6-DIHYDROXY-5-METHYLPYRIMIDINE
2,4,6-트리아이소프로필벤젠설포닐 염화물
2-METHOXYPHENOXYACETIC ACID
Fexofenadine
DI-TERT-BUTYLDICHLOROSILANE
이소펜탄
1-브로모-4-메톡시부탄
2-AMINOPENT-4-YNENITRILE
시클로부탄올
TERT-BUTYL 4-FORMYLPYRIDIN-3-YLCARBAMATE
시클로펜탄
ETHYL 1-TERT-BUTYL-3-(TRIFLUOROMETHYL)-1H-PYRAZOLE-4-CARBOXYLATE
2-(4-브로모페닐)-2-메틸프로피온산
(TRIMETHYLSILYL)ACETIC ACID
4-브로모-1-메틸-3-(트리플루오로메틸)-1H-피라졸
1-TERT-BUTYL-4-BROMO-3-(TRIFLUOROMETHYL)-1H-PYRAZOLE
(S)-2-Methylproline
클로로메틸메틸술파이드
펜탄 공급 업체
글로벌( 470)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 |
catherine@yjchem.com.cn | China | 983 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21666 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8619521488211 |
info@longyupharma.com | China | 2534 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49392 | 58 |
| BEYOND INDUSTRIES (CHINA) LIMITED | +86-21-52699951; +8613917686115 |
sales@beyondindustriesgroup.com | China | 699 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12465 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 |
ada@ipurechemical.com | China | 10326 | 58 |










