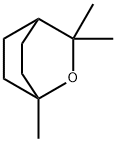
1,8-시네올
|
|
1,8-시네올 속성
- 녹는점
- 1-2 °C(lit.)
- 끓는 점
- 176-177 °C(lit.)
- 밀도
- 0.9225
- 증기압
- 1.22hPa at 20℃
- 굴절률
- n
20/D 1.457(lit.)
- FEMA
- 2465 | EUCALYPTOL
- 인화점
- 122 °F
- 저장 조건
- 2-8°C
- 용해도
- 3.5g/L
- 물리적 상태
- 액체
- 색상
- 무색투명~미황색
- 냄새
- 디프로필렌 글리콜 중 10.00%. 유칼립투스 허브 장뇌 약용
- ?? ??
- 약초
- 수용성
- 물에 용해됨(3500mg/L(21°C에서). 에테르, 알코올, 클로로포름, 빙초산, 오일과 혼합됨. 에탄올, 에틸 에테르에 용해됨, 사염화탄소에는 약간 용해됨.
- 어는점
- min. 1.4 ℃
- JECFA Number
- 1234
- Merck
- 14,3895
- BRN
- 105109
- Dielectric constant
- 4.8399999999999999
- 안정성
- 안정적인. 가연성. 산, 염기, 강산화제와 호환되지 않습니다.
- InChIKey
- WEEGYLXZBRQIMU-WAAGHKOSSA-N
- LogP
- 3.4
- CAS 데이터베이스
- 470-82-6(CAS DataBase Reference)
- NIST
- Eucalyptol(470-82-6)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 10-37/38-41-36/37/38 | ||
| 안전지침서 | 26-39-16 | ||
| 유엔번호(UN No.) | UN 1993 3/PG 3 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | OS9275000 | ||
| TSCA | Yes | ||
| HS 번호 | 2932 99 00 | ||
| 위험 등급 | 3 | ||
| 포장분류 | III | ||
| 유해 물질 데이터 | 470-82-6(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 2480 mg/kg | ||
| 기존화학 물질 | KE-34618 |
1,8-시네올 C화학적 특성, 용도, 생산
순도시험
(1) 비중 : 이 품목의 비중은 0.921~0.924이어야 한다.
(2) 선광도 : 이 품목의 선광도 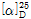 =-0.5~+0.5°이어야 한다.
=-0.5~+0.5°이어야 한다.
(3) 굴절률 : 이 품목의 굴절률 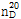 은 1.455~1.460이어야 한다.
은 1.455~1.460이어야 한다.
(4) 용상 : 이 품목 1mL를 60% 에탄올 5mL에 녹일 때, 그 액은 징명하여야 한다.
(5) 레소르신 : 이 품목 1mL를 물 5mL 및 질산제이수은시액 1mL와 물 3mL의 혼액 1방울을 넣어 잘 흔들어 섞으면서 수욕 중에서 2분간 가열한 후 식히고 이에 묽은황산 1방울 및 아질산나트륨용액 1방울을 넣어 다시 수욕 중에서 2시간 가열할 때, 물층은 황~황갈색을 나타내서는 아니 된다.
(6) 펠란스렌 : 이 품목 2.5mL에 헥산 5mL를 넣어 녹이고 이에 아질산나트륨용액(1→20) 10mL를 넣어 서서히 빙초산 5mL를 넣을 때, 10분 이내에 결정이 나타나서는 아니 된다.
정 량 법 직경 약 15mm, 길이 약 80~160mm의 시험관 (A)에 이 품목 3g 및 가온하여 녹인 o-크레솔 2.1g을 넣어 온도계 (B)를 수은구가 액중심보다 약간 밑에 가도록 코르크마개로 고정한 다음 온도계로 액을 조용히 섞어 결정이 생기기 시작하는 온도를 읽는다. A를 가열하여 결정을 완전히 용해한 다음 코르크마개 (C)를 부착한 병 (D)에 넣어 온도를 서서히 떨어뜨린다. 다시 결정이 생기기 시작하거나 또는 최초에 기록한 온도에 달할 때, 격렬히 온도계를 상하로 하여 관벽을 마찰하면 온도는 약간 떨어지는데 잠시 후에 일정온도를 나타낸다. 이 때 온도계에 나타난 온도를 읽는다. 똑같은 조작을 반복하여 얻은 온도 중 최고온도로서 다음 표에 의해 유칼리프톨의 함량을 계산한다.
확인시험
이 품목 3g에 가온하여 녹인 o-크레솔 2g을 넣어 흔들어 섞으면 결정성 덩어리로 되고 수욕 중에서 가온하면 녹는다.
화학적 성질
1,8-Cineole occurs in many terpene-containing essential oils, sometimes as the main component. For example, eucalyptus oils contain up to 85% 1,8-cineole and laurel leaf oil contains up to 70%. It is a colorless liquid with a characteristic odor, slightly reminiscent of camphor. 1,8-Cineole is one of the few fragrance materials that is obtained exclusively by isolation from essential oils, especially eucalyptus oils. Technical-grade 1,8- cineole with a purity of 99.6–99.8% is produced in large quantities by fractional distillation of Eucalyptus globulus oil. A product essentially free from other products can be obtained by crystallization of cineole-rich eucalyptus oil fractions출처
Its name is derived from its presence in the essential oils of Eucalyptus globulus and Melaleuca leucadendron L. (essential oil of cajeput). It was originally identified in the essential oil of Artemisia maritime and subsequently in a large number (approx. 270) of other essential oils: rosemary, laurel leaves, clary sage, myrrh, cardamom, star anise, camphor, lavender, peppermint, Litsea guatemalensis, Luvunga scadens Roxb., Achillea micrantha and Salvia triloba. The essential oil of Eucalyptus polibrac tea has been reported to contain up to 91% eucalyptol. Also reported found in citrus oils and juices, guava, papaya, cinnamon bark, root and leaf, ginger, corn mint oil, spearmint, nutmeg, pepper, Thymus zygis, cardamom, cranberry, laurel, pepper, sweet marjoram, coriander, Spanish origanum, Ocimum basilicum, curcuma, sage, laurel, sweet and bitter fennel, myrtle leaf and berry, pimento and calamus.용도
1,8-Cineol is the chief constituent of oil of eucalyptus. Used as pharmaceutic aid (flavor).제조 방법
By fractional distillation (170 to 180°C) from those essential oils containing high levels of eucalyptol, such as Eucalyptus globulus (approx. 60%), and subsequent separation of the product by congealing the distillate.일반 설명
Colorless liquid with a camphor-like odor. Spicy cooling taste.공기와 물의 반응
Highly flammable. Insoluble in water.반응 프로필
Cineole will react with acids and bases.화재위험
Flash point data for Cineole are not available but Cineole is probably combustible.Anticancer Research
A statistically significant reduction of cell proliferation was observed compared tothe control cells when tested on RKO cells and human colon cancer cell linesHCT116 injected into the SCID mice. The 1,8-cineole induced apoptosis by inactivatingsurviving and Akt, activating p38, inducing PARP, and cleaving caspase-3(Rodd et al. 2015).Toxicology
Eucalyptol, a colourless organic compound, is a monoterpenoid and an ether. Among its various uses, eucalyptol is predominantly used as an insecticide and in fragrances (Klocke et al. 1987). The ques- tion of whether eucalyptol could potentiate toxicity has been assessed because of its widespread use in households. When Kunming mice received feed with a high dose of eucalyptol, liver and kidney tissue demonstrated vacuolar and granular degeneration (Xu et al. 2014). When the animals were fed with a subacute dose of eucalyptol, no discernible difference in body weight was observed (Xu et al. 2014). Eucalyptol’s safety profile has been assessed. Fatality after ingestion of eucalyptol oil has been reported; the approximate lethal dose of eucalyptol in the human is between 0.05 and 0.5 mL/kg of body weight (Hindle 1994). When male rats received eucalyptol, eosinophilic protein droplets accumulated in a dose- dependent fashion (Kristiansen and Madsen 1995). However, in a study of mutagenicity using CHO cells, eucalyptol did not induce mutagenicity as evidenced in the sister chromatid exchange assay (Galloway et al. 1987). Likewise, eucalyptol did not induce carcinogenicity in pathogen-free CFLP mice (Roe et al. 1979).신진 대사
Eucalyptol undergoes oxidation in vivo with the formation of hydroxy cineole, which is excreted as hydroxycineoleglucuronic acid (Williams, 1959).Purification Methods
Purify 1,8-cineol by dilution with an equal volume of pet ether, then saturate with dry HBr. The precipitate is filtered off, washed with small volumes of pet ether, then cineole is regenerated by stirring the crystals with H2O. It can also be purified via its o-cresol or resorcinol addition compounds. Store it over Na until required. Purify it also by fractional distillation. It is insoluble in H2O but soluble in organic solvents. [IR: Kome et al. Nippon Kagaku Zasshi [J Chem Soc Japan (Pure Chem Sect)] 80 66 1959, Chem Abstr 603 1961, Beilstein 17 II 32, 17/1 V 273.]1,8-시네올 준비 용품 및 원자재
원자재
준비 용품
1,8-시네올 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Chengdu GLP biotechnology Co Ltd | 028-87075086 13350802083 |
scglp@glp-china.com | CHINA | 1824 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2931 | 58 |
| Chengdu Biopurify Phytochemicals Ltd. | +8618080483897 |
sales@biopurify.com | China | 3424 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 |
sales@finerchem.com | China | 2967 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8823 | 58 |