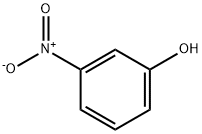
3-Nitrophenol C화학적 특성, 용도, 생산
화학적 성질
yellow to brown crystalline powder
용도
3-Nitrophenol is a chemical reagent used in various organic syntheses. Used in the synthesis of RAGE inhibitors via the etherification of phenols. Also used in the synthesis of kinase inhibitors again
st epidermal growth factor receptor T790M mutation.
생산 방법
3-Nitroaniline is diazotized in aqueous sulfuric acid and then hydrolyzed by being added gradually to boiling dilute sulfuric acid. The crude product solidifies on cooling and is filtered off in 90 % yield.
정의
ChEBI: A member of the class of 3-nitrophenols that is phenol in which one of the hydrogens that is meta to the hydroxy group has been replaced by a nitro group.
일반 설명
Colorless to pale yellow crystalline solid. Sinks in and mixes with water.
공기와 물의 반응
Water insoluble.
반응 프로필
3-Nitrophenol is a light-yellow, crystalline material, toxic and irritant. When heated to decomposition 3-Nitrophenol emits toxic fumes of oxides of nitrogen [Lewis, 3rd ed., 1993, p. 941]. Phenols do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases.
위험도
Toxic by ingestion.
건강위험
INHALATION: Inhalation or ingestion causes headaches, drowsiness, nausea, and blue color in lips, ears, and finger nails (cyanosis). EYES: Contact with eyes causes irritation. SKIN: Can be absorbed through intact skin to give same symptoms as for inhalation.
화재위험
Special Hazards of Combustion Products: Dangerous toxic fumes of NO x
Safety Profile
Poison by ingestion,
subcutaneous, and intraperitoneal routes.
Moderately toxic by skin contact. A skin and
severe eye irritant. When heated to
decomposition it emits toxic fumes of NOx.
See also other nitrophenol entries.
Purification Methods
Crystallise 3-nitrophenol from water, CHCl3, CS2, EtOH or pet ether (b 80-100o), and dry it under vacuum over P2O5 at room temperature. It can also be distilled at low pressure. The 4-nitrobenzoate had m 174o (from EtOH). [Beilstein 6 IV 1269.]
3-Nitrophenol 준비 용품 및 원자재
원자재
준비 용품