엔도술판 C화학적 특성, 용도, 생산
개요
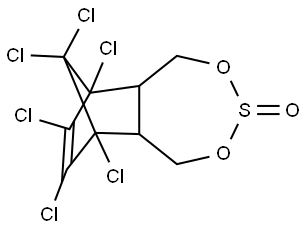
Endosulfan (70) [115-29-7], 6,7,8,9,10,10-
hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3,-
benzo-dioxathiepine 3-oxide (IUPAC) [The technical product
is a mixture of two isomers: α-endosulfan: 3α,5αβ,6α,
9α,9αβ (64–67%) (70a) and β-endosulfan 3α,5aα,6β,9β,
9aα, (29–32%) (70b)]; [959-98-8] (formerly [33213-66-
0]) (β-endosulfan);[33213-65-9] (formerly [891-86-1] and
[19670-15-6]) (β-endosulfan) is the adduct of hexachlorocyclopentadiene
and 1,4-dihydroxy-2-butene reacted further
with SOCl2 to produce 6,7,8,9,10,10-hexachloro-
1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benzodioxa
thiepin-3-oxide. The technical product is a brownish solid,
mp 70–100 ?C, vapor pressure 1.3 mPa at 25 ?C, soluble in
petroleum solvents but having low solubility in water. It
consists of about four parts of α-isomer (mp 108 ?C, cis with
regard to the sulfite group) and one part of the β-isomer
(mp 206 ?C, trans with regard to the sulfite group). The
α-isomer, which is somewhat more insecticidal, is slowly
converted to the more stable β-isomer at high temperature,
and both isomers are oxidized slowly to endosulfan sulfate
[1031-07-8] (mp 181 ?C). In acid media, both isomers form
endosulfan diol [2157-19-9] (mp 203 ?C).
화학적 성질
(commercial product): Brown crystals.
Mixture of two isomers.
용도
Insecticide.
정의
ChEBI: A cyclic sulfite ester that is 1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benzodioxathiepine 3-oxide substituted by chloro groups at positions 6, 7, 8, 9, 10 and 10.
일반 설명
Endosulfan is a pesticide. It is a cream- to brown-coloured solid that may appear in the form of crystals or flakes. It has a smell like turpentine, but does not burn. It does not occur naturally in the environment. It is a restricted use pesticide, meaning that it can only be used by professional applicators.
공기와 물의 반응
Sightly soluble in water. Slowly hydrolyzes to form sulfur dioxide and a diol; hydrolyzes more rapidly under basic or acidic conditions.
반응 프로필
Thiosulfan is an organochlorine, cyclodiene derivative. Thiosulfan is also a sulfite ester. Halogenated aliphatic or cyclic alkane compounds are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. As Thiosulfan is rather highly substituted Thiosulfan may be resistant to reaction. However, materials in this group are incompatible with strong oxidizing and reducing agents. Also, they may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. As an ester, Thiosulfan will hydrolyze to form sulfur dioxide and diol; reaction is more rapid under basic conditions.
위험도
Toxic by ingestion, inhalation, and skin
absorption; use may be restricted. Lower respiratory tract irritant; liver and kidney damage. Questionable carcinogen.
건강위험
Thiosulfan is very toxic. The probable oral lethal dose is 50 to 500 mg/kg, or 1 teaspoonful to 1 ounce for a 150 lb. person.
화재위험
Container may explode in heat of fire. Fire or run off from fire control water may release irritating or poisonous gases. Slowly oxidizes in air. Do not store at temperature below 20F.
농업용
Insecticide, Acaricide: A U.S. EPA restricted Use Pesticide (RUP). Not
approved for use in EU countries. Globally banned
as of April 29, 2010. Endosulfan was added to the list
of Stockholm Convention Persistent Organic Pollutants
(POPs): Annex A (Elimination). Endosulfan is a chlorinated
hydrocarbon insecticide and acaricide of the cyclodiene
subgroup which acts as a poison to a wide variety of
insects and mites on contact. Although it may also be used
as a wood preservative, it is used primarily on a wide variety
of food crops including tea, coffee, fruits, and vegetables,
as well as on rice, cereals, maize, sorghum, or other grains.
Formulations of endosulfan include emsulsifiable concentrate,
wettable powder, ultra-low volume (ULV) liquid, and
smoke tablets. It is compatible with many other pesticides
and may be found in formulations with dimethoate, malathion,
methomyl, monocrotophos, pirimicarb, triazophos,
fenoprop, parathion, petroleum oils, and oxine-copper. It
is not compatible with alkaline materials. Technical endosulfan
is made up of a mixture of two molecular forms
(isomers) of endosulfan, the alpha-and beta-isomers.
상품명
AFIDEN®; BEOSIT®; BIO 5,462®;
CHLORTHIEPIN®; CLEAN-CROP®; CRISUFAN®;
CYCLODAN®; DE-PESTER®; DESTROY®;
DEVISULPHAN®; DISSULFAN CE®; ENDOCEL®
ENDOCIDE®; ENDOSOL®; END-O-SULFAN®;
ENDOTAF®; ENDOX®; ENSURE®; E-Z FLO®;
FMC 5462®; HEXASULFAN®; HILDAN®; HOE
2671®; INSECTO®; INSECTOPHENE®; KENDAN®;
KERNTOX®; KOP-THIODAN®; MALIX®; MALUX;
MAUX®; MOS-570; METHOFAN®; NCI-C00566;
NIA 5462®, NIAGARA 5,462; NIAGARA 5,462®[C];
PHASER®; RASAYANSULFAN; ROCKY®;
THIFOR®; THIDAN®; THIMUL®; THIODAN®;
α-THIODAN®; β-THIODAN®; THIONEX; α-THIONEX®;
β-THIONEX®; THIOKILL®; THIOFOR®;
THIONEX®; THIOSULFAN®; THIOSULFAN
THIONEL®; THISULFAN TIOVEL; TIONEL;
TIOVEL®
Pharmacology
The rat LD
50 values are 43, 18 mg/kg (oral) and 130,
74 mg/kg (dermal). The α-isomer has somewhat greater
insecticidal activity and is slowly converted to the more
stable β-isomer at a high temperature. Both isomers
oxidize slowly in air and in biological systems to endosulfan
sulfate [1031-07-8], mp 181–182 ?C. In acid media, both
isomers form endosulfan diol [2157-19-9], mp 203–205 ?C.
Endosulfan is a broad-spectrum insecticide used to control
pests of vegetables, fruit, field crops, and ornamentals.
Unlike other cyclodiene insecticides, it is biodegradable by
hydrolysis at the sulfite ester bonds and is more readily
metabolized. It is also less persistent on plant surfaces,
and 50% of the residues are lost in 3–7 days. Volatilization
may be the major route of loss.
Endosulfan is readily hydrolyzed in water to the diol
(74), but it is moderately persistent in soil. Endosulfan (α-
and β-endosulfan) is degraded in soil with DT
50 30 to 70
days. The major metabolite is usually endosulfan sulfate
(71), which is degraded more slowly. In the field DT
50
for total endosulfan (α- and β-endosulfan and endosulfan
sulfate) is 5 to 8 months.
Safety Profile
Poison by ingestion,
inhalation, skin contact, intraperitoneal, and
subcutaneous routes. Experimental
teratogenic and reproductive effects.
Questionable carcinogen with experimental
tumorigenic and neoplastigenic data. Human
systemic effects: convulsions, cyanosis.
Human mutation data reported. A central
nervous system stimulant producing
convulsions. A htghly toxic organochlorine
pesticide that does not accumulate
significantly in human tissue. Absorption is
normally slow, but is increased by alcohols,
oil, and emulsifiers. When heated to
decomposition it emits toxic fumes of Cl
and SOx. See also CHLORIDES and
SULFITES
잠재적 노출
Those engaged in the manufacture,
formulation, and application of this material
Carcinogenicity
Equivocal results have been found in genotoxic
assays, but endosulfan was mutagenic and
clastogenic and induced effects on cell cycle
kinetics in various in vivo and in vitro tests.
In reproductive studies, male rats treated
at 3.0 mg/kg from day 15 to 21 of gestation had
reduced sperm production in adulthood.
The 2003 ACGIH threshold limit
value-time-weighted average (TLV-TWA) is
0.1mg/m3 with a notation for skin absorption.
신진 대사 경로
When endosulfan is incubated with microorganisms,
endosulfan is extensively degraded in nitrogen-
deficient, carbon-deficient, and nitrogen-rich cultures of
Phanerochaete chrysosporium and is primarily
oxidized to endosulfan sulfate, which is a terminal end
product, or hydrolyzed to the non-sulfur-containing
metabolites. An initial hydrolysis of endosulfan results
in the formation of the intermediate metabolite or
endosulfan diol, which further undergoes oxidation to
yield endosulfan hydroxyether followed by the
formation of endosulfan lactone or tentatively identified
endosulfan dialdehyde.
운송 방법
UN2761 Organochlorine pesticides, solid, toxic,
Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1;
Labels: 6.1-Poisonous materials, Technical Name Required.
비 호환성
Those engaged in the manufacture,
formulation, and application of this material
폐기물 처리
A recommended method for
disposal is burial 18 in deep in noncropland, away from
water supplies, but bags can be burned. Large quantities
should be incinerated at high temperature in a unit with
effluent gas scrubbing. Consult with environmental regulatory agencies for guidance on acceptable disposal practices.
Generators of waste containing this contaminant (≥100 kg/
mo) must conform with EPA regulations governing storage,
transportation, treatment, and waste disposal. In accordance
with 40CFR165, follow recommendations for the disposal
of pesticides and pesticide containers. Must be disposed
properly by following package label directions or by contacting your local or federal environmental control agency,
or by contacting your regional EPA office.
엔도술판 준비 용품 및 원자재
원자재
준비 용품