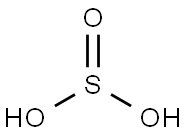
아황산
|
|
아황산 속성
- 밀도
- 1.03 g/mL at 25 °C(lit.)
- 증기 밀도
- 2.3 (vs air)
- 용해도
- solution of SO2 in H2O
- 산도 계수 (pKa)
- 1.92(at 25℃)
- 물리적 상태
- 액체
- 색상
- 무색의
- 수용성
- 물과 섞일 수 있습니다.
- 감도
- Air Sensitive
- Merck
- 14,8976
- Dielectric constant
- 15.6(0℃)
- 안정성
- 안정적인. 강한 염기와 호환되지 않습니다.
- InChIKey
- LSNNMFCWUKXFEE-UHFFFAOYSA-N
- LogP
- -1.579 (est)
- CAS 데이터베이스
- 7782-99-2(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 20-34-36/38 | ||
| 안전지침서 | 26-36/37/39-45 | ||
| 유엔번호(UN No.) | UN 1833 8/PG 2 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | WT2775000 | ||
| F 고인화성물질 | 10-23 | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | II | ||
| HS 번호 | 28111990 | ||
| 기존화학 물질 | KE-32671 |
아황산 C화학적 특성, 용도, 생산
개요
Sulfurous Acid is a weak and unstable inorganic acid, which is considered an aqueous solution of sulfur dioxide in water. It is formed theoretically by burning sulfur to produce sulfur dioxide, which is then reacted with water. However, there is no evidence that sulfurous acid exists in solution, while the molecules of which has been detected in the gas phase, since the reaction is reversible and the acid readily decomposes back into the reactants. Sulfurous acid is not usually available in its acid form, but more commonly prepared as its sodium or potassium salts.Sulfurous acid and its salts are commonly applied as powerful reducing agents and disinfectant agents due to its strong reducibility. It is also considered as a mild bleaching agent especially for applications having chlorine sensitive materials. Besides, Sulfurous acid is a food additive listed on the EAFUS food Additive Database (Jan. 2001).
화학적 성질
SULFUROUS ACID, colorless liquid, prepared by dissolving SO2 in H2O. Reagent grade H2SO3 contains approximately 6% SO2 in solution용도
Sulfurous acid (H2SO3) can be produced by burning sulfur to form sulfur dioxide (SO2) gas and by then dissolving the gas in water to form sulfurous acid. This is the acid produced by burning coal that has a high sulfur content; the gaseous sulfur dioxide by-product of combustion then combines with atmospheric water to form “acid rain.”정의
A weak acid found only in solution, made by passing sulfur(IV) oxide into water. The solution is unstable and smells of sulfur(IV) oxide. It is a reducing agent, converting iron(III) ions to iron(II) ions, chlorine to chloride ions, and orange dichromate(VI) ions to green chromium(III) ions.일반 설명
Sulfurous acid, H2S03, is an unstable, water-soluble, colorless liquid with a pungent burning sulfur odor. Corrosive to metals and tissue. It is derived from absorption of sulfur dioxide( S02) in water.Reagent grade sulfurous acid contains about 6% sulfur dioxide in solution. Sulfurous acid is a strong reducing agent.It is readily oxidized to sulfuric acid and is itself reduced to hydrogen sulfide by zinc and dilute sulfuric acid. Sulfurous acid is used in the synthesis of medicines and chemicals, in the manufacture of paper and wine, in brewing, in metallurgy, in ore flotation, as a bleach and analytical reagent, and for refining of petroleum products.공기와 물의 반응
Soluble in water with release of heat.반응 프로필
Colorless, corrosive, moderately toxic liquid. When heated to decomposition SULFUROUS ACID emits toxic fumes of oxides of sulfur [Lewis, 3rd ed., 1993, p. 1196].위험도
Toxic by ingestion and inhalation, strong irritant to tissue.건강위험
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.화재위험
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.공업 용도
Sulfurous acid (H2SO3) is usually marketed as liquid SO2. The bulk of SO2 is produced from off-smelter gases. Although handling of SO2 liquid requires special equipment, it is frequently used as a pH regulator and depressant, primarily during the treatment of complex sulfide ores. SO2 is largely used in North American operations as a pyrite depressant and for the depression of galena during copper/lead separation.Safety Profile
A poison by ingestion and inhalation. A corrosive irritant to skin, eyes, and mucous membranes. Human systemic effects by ingestion: nausea or vomiting, hypermotility, diarrhea, and othergastrointestinal effects. When heated to decomposition it emits highly toxic fumes ofSOx.참고 문헌
https://en.wikipedia.org/wiki/Sulfurous_acidhttp://printwiki.org/Sulfurous_Acid
http://www.softschools.com/formulas/chemistry/sulfurous_acid_uses_properties_structure_formula/242/
https://pubchem.ncbi.nlm.nih.gov/compound/Sulfurous_acid#section=Top
http://www.chemistrylearner.com/sulfurous-acid.html
아황산 준비 용품 및 원자재
원자재
준비 용품
시안화 제I구리
LAMBDACYHALOTHRIN 테크니컬
신남알데히드
polymer bactericidal flocculent
p-멘타-6,8-다이엔-2-온, (R)-(-)
whitener WG for wool
sulfonated ligno-sulfomethylated phenolic resin copolymer
3-인다졸리논
타우린
hydrogen [4-[[4-(diethylamino)phenyl][4-[ethyl[(3-sulphonatobenzyl)amino]-o-tolyl]methylene]-3-methylcyclohexa-2,5-dien-1-ylidene](ethyl)(3-sulphonatobenzyl)ammonium, sodium salt
Direct Fast Yellow RR
바나듐 황산염
2,2'-(1,2'-에텐다이일)비스[5-((4-에톡시페닐)아조]벤젠설포닉애씨드) 및 그 염류
Brinzolamide
2,4,6-트리클로로페놀
DIRECT YELLOW 27
파클로부트라졸
sopa
(Strontium-Calcium) Chrome Yellow
Benazepril
하이드록시시트로넬랄
흑색 168 염료
DL-트립토판
DIRECT VIOLET 51
(4S)-HYDROXY-3-METHYL-2-(2-PROPENYL)-2-CYCLOPENTENE-1-ONE
DL-Arginine hydrochloride monohydrate
sulfo-imidazoline betaine
호모피페라진
염화구리(Ⅰ)
티펜술퍼론-메틸
1-Boc-hexahydro-1,4-diazepine
인디고 카르민
바닐린
시트랄
6-메틸 벤조피론
Deemulsifier SP-169
2-Hydroxy-6-chloroquinoxaline
Ambroxol
아황산 공급 업체
글로벌( 205)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Qingdao kaimoshi biochemical technology co., ltd. | +86-0571-87191913 +86-19957014543 |
info@kaimosi.com | CHINA | 144 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 |
eric@witopchemical.com | China | 23556 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 9320 | 58 |
| XINGTAI XINGJIU NEW MATERIAL TECHNOLOGY CO., LTD | +8617731987558 |
xingjiu@xingjiubiotech.com | China | 990 | 58 |
| Hebei Duling International Trade Co. LTD | +8618032673083 |
sales05@hbduling.cn | China | 15745 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503259 +86-19930503259 |
cherry@crovellbio.com | China | 18456 | 58 |