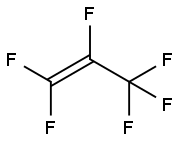
헥사플루오로프로필렌
|
|
헥사플루오로프로필렌 속성
- 녹는점
- −153 °C(lit.)
- 끓는 점
- −28 °C(lit.)
- 밀도
- 1,583 g/cm3
- 증기압
- 587.952kPa at 25℃
- 굴절률
- 1.4000
- 물리적 상태
- 가스
- 수용성
- 82mg/L at 28℃
- BRN
- 1705671
- 안정성
- 안정적인. 알칼리 금속, 분말 금속과 호환되지 않습니다.
- InChIKey
- HCDGVLDPFQMKDK-UHFFFAOYSA-N
- LogP
- 1.95 at 20℃
- CAS 데이터베이스
- 116-15-4(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 20-37-68/20-48/20 | ||
| 안전지침서 | 41-36/37 | ||
| 유엔번호(UN No.) | UN 1858 2.2 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | UD0350000 | ||
| F 고인화성물질 | 4.5 | ||
| 위험 참고 사항 | Irritant | ||
| 위험 등급 | 2.2 | ||
| HS 번호 | 2903590030 | ||
| 유해 물질 데이터 | 116-15-4(Hazardous Substances Data) | ||
| 독성 | LC50 inhalation in mouse: 750ppm/4H | ||
| 기존화학 물질 | KE-18543 |
헥사플루오로프로필렌 C화학적 특성, 용도, 생산
화학적 성질
Hexafluoropropylene (HFP) is a colourless, odourless, non-flammable gas that is only slightly soluble in water. It is used as chemical intermediate primarily in the synthesis of fluoropolymers, fluoro-elastomers and fluorinated materials (e.g. perfluoropolyether functional fluids, oils and greases).용도
Hexafluoropropene is used in the synthesis of polyvinylidene fluoride-co-hexafluoropropylene, a polymer which is currently being used in various fields of the nano and electronic sector ranging from membranes to nanoparticles.제조 방법
Hexafluoropropylene can be produced by pyrolysis of tetrafluoroethylene (TFE):3 CF2=CF2 → 2 CF3CF=CF
This reaction is carried out in a closed continuous reactor at 600 to 900°C. The yield can be improved at low partial pressure, achieved either by operating under reduced total pressure (down to 0.1 bar) or by injecting an inert diluent such as steam or CO2.
Significant quantities of by-products, including the highly toxic perfluoroisobutylene (PFIB), are formed during the pyrolysis of TFE. Such by-products are either incinerated directly or are collected and transported for incineration at a remote facility.
Hexafluoropropylene can also be prepared from chlorodifluoromethane, or produced from various chlorofluorocarbons.
일반 설명
Hexafluoropropylene is an odorless, colorless gas. Hexafluoropropylene is noncombustible. Hexafluoropropylene can asphyxiate by the displacement of air. Exposure of the container to prolonged heat or fire can cause Hexafluoropropylene to rupture violently and rocket.반응 프로필
Halogenated aliphatic compounds, such as Hexafluoropropylene, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Materials in this group may be incompatible with strong oxidizing and reducing agents. Also, they may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. Above a minimum oxygen pressure, the reaction of oxygen difluoride and hexafluoropropene to yield the Hexafluoropropylene oxide becomes explosive, Chem. Abs., 1987, 107, 175302. The reaction of hexafluoropropene with grignard reagent (subst. phenylmagnesium bromides) led to explosion, Fluorine Chem., 1981, 18, 25.건강위험
Vapors may cause dizziness or asphyxiation without warning. Vapors from liquefied gas are initially heavier than air and spread along ground. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating, corrosive and/or toxic gases.화재위험
Some may burn but none ignite readily. Containers may explode when heated. Ruptured cylinders may rocket.헥사플루오로프로필렌 준비 용품 및 원자재
원자재
준비 용품
1,1,1,2,3,3,3-헵타플루오로프로판
테트라플루오르에틸렌-헥사플루오르프로필렌 공중합체
(3,3,3-트라이플루오로프로필)트라이페톡시실레인
Heptafluoroisopropyl iodide
BIS(HEPTAFLUOROISOPROPYL)KETONE
1,1,2,3,3,3-HEXAFLUOROPROPYL 2,2,2-TRIFLUOROETHYL ETHER
헵타 플루오로-1-메톡시 프로판
1H,1H,2'H-PERFLUORODIPROPYL ETHER
1,1,2,3,3,3-HEXAFLUOROPROPYL METHYL ETHER
PERFLUORO(2-METHYLPROPANOYL)FLUORIDE
과불화프로판
1,2-디브로모-1,1,2,3,3,3-헥사플루오로프로판
헥사플루오로프로필렌 공급 업체
글로벌( 161)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1807 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5909 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49390 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; |
factory@coreychem.com | China | 29826 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81148696 +8615536356810 |
1047@dideu.com | China | 3408 | 58 |
| BEYOND INDUSTRIES (CHINA) LIMITED | +86-21-52699951; +8613917686115 |
sales@beyondindustriesgroup.com | China | 699 | 58 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17367 | 58 |
헥사플루오로프로필렌 관련 검색:
프로필렌 헥사플루오로프로펜 산화물 아크릴아마이드 헥사플루오로에테인(헥사플루오르에탄) 프로필렌 카보네이트 폴리프로필렌 알릴 알코올 아크롤레인 테트라플루오르에틸렌-헥사플루오르프로필렌 공중합체 헥사플루오르프로필렌 에폭시드의 불소 말단-차폐된 호모중합체 헥사플루오르프로필렌-비닐리덴 플루오리드 공중합체 헥사플루오로프로필렌
Cefprozil hydrate
2,2,3,4,4,4-Hexafluorobutyl methacrylate
2,2,3,4,4,4-Hexafluorobutyl acrylate
Cefprozil
Sodium hexafluoroantimonate
Calcium hexafluorophosphate