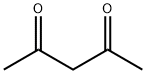
2,4-펜탄디온
|
|
2,4-펜탄디온 속성
- 녹는점
- -23 °C (lit.)
- 끓는 점
- 140.4 °C (lit.)
- 밀도
- 0.975 g/mL at 25 °C (lit.)
- 증기 밀도
- 3.5 (vs air)
- 증기압
- 6 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.452(lit.)
- 인화점
- 66 °F
- 저장 조건
- Store below +30°C.
- 용해도
- H2O: 용해성1 8부
- 산도 계수 (pKa)
- 8.9(at 25℃)
- 물리적 상태
- 액체
- 색상
- 매우 진한 녹황색
- 상대극성
- 0.571
- 수소이온지수(pH)
- 6 (200g/l, H2O, 20℃)
- 냄새
- 기분 좋은 냄새
- 폭발한계
- 2.4-11.4%(V)
- 수용성
- 16g/100mL(20℃)
- Merck
- 14,81
- BRN
- 741937
- 노출 한도
- No exposure limit has been set.
- Dielectric constant
- 23.1(20℃)
- InChIKey
- YRKCREAYFQTBPV-UHFFFAOYSA-N
- LogP
- 0.68 at 20℃
- CAS 데이터베이스
- 123-54-6(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-36/37/38-22-10-R22-R10-20/21/22-2017/10/22 | ||
| 안전지침서 | 21-23-24/25-36-26-S24/25-S23-S21 | ||
| 유엔번호(UN No.) | UN 2310 3/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | SA1925000 | ||
| F 고인화성물질 | 9-23 | ||
| 자연 발화 온도 | 662 °F | ||
| TSCA | Yes | ||
| HS 번호 | 2914 19 90 | ||
| 위험 등급 | 3 | ||
| 포장분류 | III | ||
| 유해 물질 데이터 | 123-54-6(Hazardous Substances Data) | ||
| 독성 | LC50 (4 hrs) in rats: 1000 ppm (Carpenter) | ||
| 기존화학 물질 | KE-27993 |
2,4-펜탄디온 C화학적 특성, 용도, 생산
물성
이 제품은 무색 또는 약간 황색의 투명한 액체로 냄새가 나고, mp-23 ℃, bp140.4 ℃, n20D : 1.4520, 상대 밀도는 0.975이며 알코올, 에테르, 클로로포름, 아세톤과 섞일 수 있음. , 아세트산 및 다른 유기 용매; 또한 물에 용해된다. 이 제품은 인화성이며 부식성이 있습니다.존재
2, 4- 펜탄 디온은 파파야에서 발견됩니다. 아세틸 아세톤은 Pinus sylvestris (Scotch pine)의 미묘한 유분으로부터 분리됩니다. 2,4- 펜탄 디온은 아크릴로 일 작용기를 함유하는 유기 화합물 인 아크릴로 일 화합물 군에 속한다.화학 반응
나트륨이나 알콕시화나트륨 등 염기 존재하에 아세톤과 아세트산에틸을 축합시키거나 삼플루오르화붕소 존재하에 아세톤과 아세트산무수물을 축합시켜 만든다. 보통 케토형 CHCOCHCOCH와 에놀형 CHCOCH=C(OH)CH의 두 이성질체의 평형 혼합물로 존재하고 평형 위치는 용매의 성질에 따라 다른데 유리상태에서는 70%가 에놀형이다.개요
아세틸 아세톤은 두 가지 토토 머 형태로 존재하는 유기 화합물로, 상호 변환이 빠르고 대부분의 응용에서 단일 화합물로 취급됩니다. 화합물이 공식적으로 디케 톤 형태 인 펜탄 -2,4- 디온으로 명명되었지만, 에놀 형태는 물질의 실질적인 성분을 형성하고 실제로 많은 용매에서 선호되는 형태이다. 이것은 아세틸 아세토 네이트 (acac)의 전구체 인 무색의 액체이며, 일반적인 두자리 리간드입니다. 그것은 또한 복 소환 화합물의 합성을위한 빌딩 블록이다.용도
1. 주로 약국 및 중간체의 합성에 사용; 2. 동물 용 의약품과 사료 첨가물의 합성에 사용된다. 3. 셀룰로오스 아세테이트의 용제, 가솔린 및 윤활유 용 첨가제 및 전기 도금 용 결합제로 사용됩니다. 4. 시약 분석.포장, 보관 및 운송
발화원 및 부식성 및 반응성 물질로부터 멀리 떨어진 가연성 액체 저장 공간 또는 승인 된 캐비닛에 보관하십시오. + 5 ° C ~ + 30 ° C에서 보관하십시오.개요
Acetylacetone (2,4-pentanedione) is a clear or slightly yellowish liquid with a putrid odour. It is readily soluble in water and in organic solvents and incompatible with light, ignition sources, excess heat, oxidising agents, strong reducing agents, and strong bases. On decomposition, acetylacetone releases hazardous products such as carbon monoxide, irritating and toxic fumes and gases, and carbon dioxide. Acetylacetone is used in the production of anti-corrosion agents and its peroxide compounds for the radical initiator application for polymerisation. It is used as a chemical intermediate for drugs (such as sulphamethazine, nicarbazine, vitamin B6, and vitamin K) and pesticides sulfonylurea herbicides and pesticides. It is used as an indicator for the complexometric titration of Fe (III), for the modification of guanidino groups and amino groups in proteins, and for the preparation of metal acetylacetonates for catalyst application.화학적 성질
Acetylacetone (2,4-pentanedione) is a clear or slightly yellowish liquid with a putrid odor. It is readily soluble in water. It is with other incompatible materials, light, ignition sources, excess heat, oxidizing agents, strong reducing agents, and strong bases. On decomposition, acetylacetone releases hazardous products, such as carbon monoxide, irritating and toxic fumes and gases, and carbon dioxide. Acetylacetone is used in the production of anticorrosion agents and its peroxide compounds for the radical initiator application for polymerization. It is used as a chemical intermediate for drugs (such as sulfamethazine, nicarbazine, vitamin B6, and vitamin K), sulfonylurea herbicides, and pesticides. It is used as a solvent for cellulose acetate, as an additive in gasoline and lubricant, as a dryer of paint and varnish. It is used as an indicator for the complexometric titration of Fe(III), for the modifi cation of guanidino groups and amino groups in proteins, and in the preparation of metal acetylacetonates for catalyst application.용도
Acetylacetone was used in preparing Y203, La203 and La2CuO4 thin films and the titanate/anatase dual-phase photocatalyst.생산 방법
2,4-Pentanedione is produced by thermal or metal-catalyzed rearrangement of isopropenyl acetate(obtained from acetone and ketene):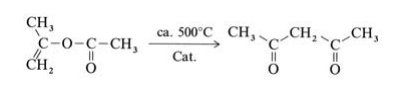
Isopropenyl acetate vapor is fed at atmospheric pressure through a V2A steel tube with an inner temperature of 520℃. The hot reaction gases are quenched, condensed, and cooled to 20℃, whereby the gaseous byproducts carbon monoxide, carbon dioxide, methane, and ketene are separated. The product is purified by fractional distillation. Other industrially less important processes for the production of 2,4-pentanedione, include the Claisen ester condensation of ethyl acetate with acetone using sodium ethoxide as condensation agent and the acetylation of acetoacetic acid esters with acetic anhydride in the presence of magnesium salts.
정의
ChEBI: A beta-diketone that is pentane in which the hydrogens at positions 2 and 4 are replaced by oxo groups.주요 응용
Acetylacetone, also known as 2,4-pentanedione, is an important commodity chemical and widely used as a fuel additive, as dyeing intermediate, in the fields of metal extraction, metal plating, and resin modification. Hantzsch reaction was used as a derivatizing agent for the assay of compounds having a primary amino group. The reagent was reacted with the primary amino group of the drugs to form a product having color and/or emit fluorescence. This condensation reaction was distinguished by its precision, reproducibility, and analytical cost reduction. FLX contains an aliphatic amino group, in the presence of formaldehyde solution, this amino group can condense with two equivalents of acetylacetone to form dihydropyridine derivative that emits yellow fluorescent product. (Figure1). Under optimized conditions of the reaction, FLX gave highly fluorescent product measured at λem 479 nm using 419 nm as excitation.일반 설명
A colorless or yellow colored liquid. Less dense than water. Flash point 105°F. Vapors are heavier than air. Used as a solvent in paints and varnishes.공기와 물의 반응
Flammable. Soluble in water.반응 프로필
Ketones, such as 2,4-Pentanedione, are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4. May dissolve plastics [USCG, 1999].건강위험
Inhalation causes dizziness, headache, nausea, vomiting and loss of consciousness. Contact with liquid irritates eyes.화재위험
Behavior in Fire: Vapor is heavier than air and may travel to a source of ignition and flash back.Safety Profile
Poison by ingestion and intraperitoneal routes. Moderately toxic by inhalation. A skin and severe eye irritant. Experimental reproductive effects. Mutation data reported. Flammable liquid when exposed to heat or flame. Incompatible with oxidning materials. To fight fire, use alcohol foam, CO2, dry chemical.잠재적 노출
Acetoacetic acid derivative. 2,4-Pentanedione is used in gasoline and lubricant additives, fungicides, insecticides, and colors manufacture; as a chemical intermediate and in the manufacture of metal chelates저장
Acetylacetone should be stored away from heat, sparks, flame, and from sources of ignition. It should be stored in a tightly sealed container, in a cool, dry, well-ventilated area, away from incompatible substances.운송 방법
UN2310 Pentane-2,4-dione, Hazard Class: 3; Labels: 3-Flammable liquidPurification Methods
Small amounts of acetic acid are removed by shaking with small portions of 2M NaOH until the aqueous phase remains faintly alkaline. The sample, after washing with water, is dried with anhydrous Na2SO4, and distilled through a modified Vigreux column (p 11) Cartledge J Am Chem Soc 73 4416 1951]. An additional purification step is fractional crystallisation from the liquid. Alternatively, there is less loss of acetylacetone if it is dissolved in four volumes of *benzene and the solution is shaken three times with an equal volume of distilled water (to extract acetic acid): the *benzene is then removed by distillation at 43-53o and 20-30mm through a helices-packed column. It is then refluxed over P2O5 (10g/L) and fractionally distilled under reduced pressure. The distillate (sp conductivity 4 x 10-8 ohm-1cm-1) is suitable for polarography [Fujinaga & Lee Talanta 24 395 1977]. To recover used acetylacetone, metal ions are stripped from the solution at pH 1 (using 100mL 0.1M H2SO4/L of acetylacetone). The acetylacetone is then washed with (1:10) ammonia solution (100mL/L) and with distilled water (100mL/L, twice), then treated as above. It complexes with Al, Be, Ca, Cd, Ce , Cu, Fe2+, Fe3+ , Mn, Mg, Ni, Pb and Zn. [Beilstein 1 H 777, 1 I 401, 1 II 831, 1 III 3113, 1 IV 3662.]비 호환성
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. reducing agents; halogens, aliphatic amines; alkanolamines, organic acids; isocyanates. Strong light may cause polymerization.폐기물 처리
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.주의 사항
Occupational workers should only use/handle acetyl acetone in a well-ventilated area, with spark-proof tools and explosion-proof equipment. Workers should not cut, weld, braze, solder, drill, grind, pressurize, or expose empty containers to heat, sparks, or flames.2,4-펜탄디온 준비 용품 및 원자재
원자재
트리에틸인산염
dilute sulphuric acid
p-클로로벤즈알데히드
아세톤
나트륨
구리 아세트산 모노수화물
이황화탄소
아세토바닐론
아세트산(빙초산)
나트륨아미드
아세트산이소프로펜일에스테르
케텐
준비 용품
테트라하이드로다이페룰로일메탄
5-CYANO-6-HYDROXY-4-METHOXYMETHYL-2-METHYLPYRIDINE
알루미늄 아세틸아세토네이트
17-Ethinyl-3,17-dihydroxy-18-methylestra-2,5(10)-diene3-methylether
닌히드린
3-(3,5-DIMETHYL-PYRAZOL-1-YL)-PROPYLAMINE
1,2-DIHYDRO-4-(METHOXYMETHYL)-6-METHYL-5-NITRO-2-OXONICOTINONITRILE
4-methoxymethylpyridoxine
2-AMINO-4,6-DIMETHYL-3-PYRIDINECARBOXAMIDE
5-BROMO-2-CHLORO-4,6-DIMETHYLNICOTINONITRILE
4,6-Dimethyl-2-methylmercapyrimidine
4,6-DIMETHYL-PYRIMIDINE-2-SULFONYL FLUORIDE
1,4,6-Trimethyl-1H-pyrazolo[3,4-b]pyridin-3-ylamine ,97%
2-(2-CARBOXYETHYL)THIO-4,6-DIMETHYLPYRIMIDINE
4,6-DIMETHYL-2-THIOPYRIMIDINE
2-(CARBOXYMETHYLTHIO)-4,6-DIMETHYLPYRIMIDINE MONOHYDRATE
13-Ethyl-17-hydroxy-18,19-dinorpregn-5(10)-en-20-yn-3-one
아세틸(5-)-2,4-디메틸티아졸
Bis(2,4-pentanedionato-O,O')palladium(II)
2-히드록시-4,6-디메틸피리미딘 염화수소산염
2-히드록시-4,6-디메틸피리딘-3-카르보니트릴
에틸(2-)-3-메틸피라지온
MEQUINDOX
6-CHLORO-5-CYANO-4-METHOXYMETHYL-3-NITRO-2-PICOLINE
17-Ethinyl-17-hydroxy-18-methylestra-5(10),9(11)-dien-3-one-3-ethylene ketal
2-Amino-4,6-dimethylpyrimidine
1-(4-METHYL-2-(METHYLTHIO)PYRIMIDIN-5-YL)ETHANONE
3-(PYRIMIDIN-2-YLTHIO)PENTANE-2,4-DIONE
1-(2,4-DIMETHYLQUINOLIN-3-YL)ETHANONE HYDROCHLORIDE
2-Chloro-3-cyano-4,6-dimethylpyridine
3,5-DIMETHYLPYRAZOLE-1-CARBOXAMIDINE NITRATE
5-AMINO-4-(METHOXYMETHYL)-6-METHYL-3-PYRIDINEMETHANAMINE
3,5-DIMETHYL-1H-PYRAZOLE-4-CARBOXYLIC ACID
설파메타진
3-ACETYL-2-METHYL-QUINOLINE-4-CARBOXYLIC ACID
5-BROMO-4,6-DIMETHYL-1H-PYRAZOLO[3,4-B]PYRIDIN-3-AMINE
4,6-Dimethyl-2-hydroxypyridine
17-Ethynyl-18-methylestra-5(10),9(11)-dien-17-ol-3-one
4,6-DIMETHYL-1H-PYRAZOLO[3,4-B]PYRIDIN-3-AMINE
2-CHLORO-3-(3,5-DIMETHYL-PYRAZOL-1-YL)-QUINOXALINE
2,4-펜탄디온 공급 업체
글로벌( 617)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Tianjin Zhongxin Chemtech Co., Ltd. | +86-022-66880623 +8618622897568 |
sales@tjzxchem.com | China | 559 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 |
ada@ipurechemical.com | China | 10326 | 58 |
| Changzhou AniKare Pharmatech Co., Ltd. | +86-0519-8359-8696 +8618018249389 |
sales@anikare.com | China | 8382 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1807 | 55 |
| Anhui Royal Chemical Co., Ltd. | +86-25-86655873 +8613962173137 |
marketing@royal-chem.com | China | 142 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Yancheng Green Chemicals Co.,Ltd | +undefined-86-25-86655873 |
info@royal-chem.com | China | 114 | 58 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 |
kaia@neputrading.com | China | 1011 | 58 |