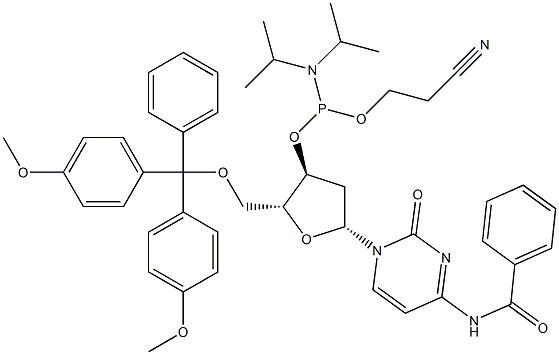
DMT-dC(bz) Phosphoramidite
|
|
DMT-dC(bz) Phosphoramidite 속성
- 녹는점
- >84°C (dec.)
- 밀도
- 1.23 at 20℃
- 증기압
- 0-0Pa at 20-50℃
- 저장 조건
- Sealed in dry,2-8°C
- 용해도
- 클로로포름, DMSO, 메탄올(약간 용해됨)
- 물리적 상태
- 분말 또는 과립
- 산도 계수 (pKa)
- 8.65±0.20(Predicted)
- 색상
- 흰색에서 황백색까지
- InChIKey
- PGTNFMKLGRFZDX-SALLYJDFSA-N
- SMILES
- O(C(C1=CC=C(OC)C=C1)(C1=CC=C(OC)C=C1)C1=CC=CC=C1)C[C@H]1O[C@@H](N2C=CC(NC(=O)C3=CC=CC=C3)=NC2=O)C[C@@H]1OP(N(C(C)C)C(C)C)OCCC#N |&1:25,27,45,r|
- LogP
- 6.5 at 20℃
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 안전지침서 | 24/25 | ||
|---|---|---|---|
| WGK 독일 | 3 | ||
| HS 번호 | 29349990 |
| 그림문자(GHS): |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Warning | ||||||||||||||
| 유해·위험 문구: |
|
||||||||||||||
| 예방조치문구: |
|
DMT-dC(bz) Phosphoramidite C화학적 특성, 용도, 생산
화학적 성질
DMT-dC(bz) Phosphoramidite, also known as 5'-O-DMT-N4-Benzoyl-2'-Deoxycytidine-CE Phosphoramidite or (2R,3R,4R,5R)-2-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-5-(2-isobutyramido-6-oxo-1,6-dihydro-9H-purin-9-yl)-4-(2-methoxyethoxy)tetrahydrofuran-3-yl (2-cyanoethyl) diisopropylphosphoramidite (IUPAC Name), is a novel nucleoside amidite analog, which can be subjected in the synthesis of DNA.DMT-dC(bz) Phosphoramidite belongs to the group of DNA Phosphoramidites.Its key features include:
Exocyclic amine functions are protected by a benzoyl group (dA(bz) anddC(bz)) or isobutyryl group (dG(ib))
Recommended cleavage and deprotection conditions are 8 hours at 55 °Cor 24 hours at room temperature using concentrated ammonia solution, for standard base-protected oligonucleotides
The high coupling efficiency of Proligo′s DNA phosphoramidites leads to high-yield and high-quality oligonucleotides.
용도
5'-O-DMT-N4-Benzoyl-2'-deoxycytidine 3'-CE phosphoramidite is used to prepare antisense oligonucleotides containing conformationally constrained methoxyaminomethylene and aminooxymethylene and aminomethylene bridged nucleoside analogs.Synthesis
These Examples illustrate the phosphitylation of several protected nucleoside reagents with 2-Cyanoethyl-N,N,N',N'-tetraisopropylphosphordiamidite in the presence of several activators according to the present invention. Eleven phosphitylation reactions (1-11) comprising reacting a protected nucleoside reagent with 2-Cyanoethyl-N,N,N',N'-tetraisopropylphosphordiamidite in the presence of an acid-base activator according to the present invention were conducted, and the product yields of each calculated, as described in the General Procedure, below. The various combinations of protected nucleoside, activator base, activator acid, solvent, and yield for each of the 11 reactions are listed in Table 1. General Procedure: The activator base (1.1 to 1.2 equivalents) is added to the solvent and 0.95 to 1.1 equivalents of activator acid is subsequently added thereto at ambient temperature to form the activator solution. About 1 equivalent of the protected nucleoside is dissolved in about 10 equivalents of the solvent in a separate vessel and about 3 equivalents of the solvent is then distilled off under reduced pressure. About 1 to 1.2 equivalents of 2-Cyanoethyl-N,N,N',N'-tetraisopropylphosphordiamidite is added to the nucleoside mixture at ambient temperature, and the activator solution prepared previously is then added to the nucleoside mixture at ambient temperature with vigorous stirring. After 12 hours, the reaction mixture is diluted with toluene and washed with water. The organic layer is separated, dried over sodium sulfate if necessary, and concentrated under reduced pressure. The yield of the desired amidite is then calculated using HPLC techniques, that is, the resulting product mixture is run through an HPLC column using an appropriate eluent, and the area under the HPLC peaks used to determine the %yield of product in the mixture.
DMT-dC(bz) Phosphoramidite 준비 용품 및 원자재
원자재
준비 용품
DMT-dC(bz) Phosphoramidite 공급 업체
글로벌( 175)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Zhejiang Hengkang Pharmaceutical Co., Ltd. | +86-576-83372028 +86-18868723926 |
bd1@hengkangpharm.cn | China | 119 | 58 |
| Chemtour Biotech Co., Ltd | +8617327281506 |
market@chemtour.com | China | 1472 | 58 |
| Hefei Huana Biomedical Technology Co.,Ltd. | +86-15900695956 |
shiqin.he@huanaok.com.cn | China | 96 | 58 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Jinan Carbotang Biotech Co.,Ltd. | +8615866703830 |
figo.gao@foxmail.com | China | 7019 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Yunbio Tech Co.,Ltd. | +86-010-60605551 +86-18046518538 |
yunbiochem@126.com | China | 321 | 58 |
| Nanjing Baifuli Technology Co., Ltd. | +86-15335185688 |
sales@unisyn.cn | CHINA | 332 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 |
sales@alchempharmtech.com | United States | 63711 | 58 |
DMT-dC(bz) Phosphoramidite 관련 검색:
시틴딘 과산화 벤조일 (3-클로로프로필)트리메톡시실란 3-(트라이에톡시실릴)프로필아민 염화벤조일 디에틸포스포노아세트산에틸에스테르 아라비노실시토신 N4-벤조일-5'-O-(4,4'-디메톡시트리틸)-2'데옥시시티딘
BENZOYL ISOCYANATE
Cytidine-5'-triphosphate disodium salt dihydrate
3-Chloropropyltriethoxysilane
2'-Deoxycytidine
N-blocked-5'-O-DMT-2'-O-Me CED cytosine phosphoramidite
5'-O-FDMT-N4-BENZOYL-2'-DEOXYCYTIDINE CEP
5-HYDROXY-DC CEP
5-BROMO-DC CEP
N-blocked-5'-O-DMT-2'-O-TBDMS CED cytidine phosphoramidite
DMT-dC(bz) Phosphoramidite