염화벤조일
|
|
염화벤조일 속성
- 녹는점
- -1 °C (lit.)
- 끓는 점
- 198 °C (lit.)
- 밀도
- 1.211 g/mL at 25 °C (lit.)
- 증기 밀도
- 4.88 (vs air)
- 증기압
- 1 mm Hg ( 32 °C)
- 굴절률
- n
20/D 1.553(lit.)
- 인화점
- 156 °F
- 저장 조건
- Store below +30°C.
- 용해도
- 아세토니트릴(약간 용해됨), 클로로포름(약간 용해됨)
- 물리적 상태
- 액체
- 색상
- 투명한
- 냄새
- 매운 특성.
- pH 범위
- 2 at 1 g/l
- 수소이온지수(pH)
- 2 (1g/l, H2O, 20℃)
- 폭발한계
- 2.5-27%(V)
- 수용성
- 물과 반응
- 어는점
- -1℃
- 감도
- Moisture Sensitive
- Merck
- 14,1112
- BRN
- 471389
- Dielectric constant
- 23.0(0℃)
- 노출 한도
- ACGIH: Ceiling 0.5 ppm
- 안정성
- 안정적인. 타기 쉬운. 강한 산화제, 물, 알코올, 강염기와 호환되지 않습니다. DMSO와 격렬하게 반응하고 알칼리와 격렬하게 반응합니다.
- InChIKey
- PASDCCFISLVPSO-UHFFFAOYSA-N
- LogP
- 1.44 at 21℃ and pH6
- CAS 데이터베이스
- 98-88-4(CAS DataBase Reference)
- IARC
- 2A (Vol. 29, Sup 7, 71) 1999
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 34-43-20/21/22 | ||
| 안전지침서 | 26-45-36/37/39 | ||
| 유엔번호(UN No.) | UN 1736 8/PG 2 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | DM6600000 | ||
| 자연 발화 온도 | 600 °C | ||
| 위험 참고 사항 | Corrosive | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | II | ||
| HS 번호 | 29310095 | ||
| 유해 물질 데이터 | 98-88-4(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 2460 mg/kg LD50 dermal Rabbit 790 mg/kg | ||
| 기존화학 물질 | KE-02765 | ||
| 중점관리물질 필터링 | 별표1-45 |
염화벤조일 C화학적 특성, 용도, 생산
개요
벤조산의 산염화물. 자극적인 냄새가 나는 무색 액체로, 에테르에 녹고 물에서 분해된다.용도
벤조일화제(benzoyl化劑)로 널리 쓴다.화학적 성질
Benzoyl chloride is a colorless to slight brown liquid with a strong, penetrating odor; vapor causes tears. Soluble in ether and carbon disulfide; decomposes in water. Combustible. It is a liquid acyl chloride used as a benzoylating agent.생산 방법
Benzoyl chloride can be prepared from benzoic acid by reaction with PCl5 or SOCl2, from benzaldehyde by treatment with POCl3 or SO2 Cl2, from benzotrichloride by partial hydrolysis in the presence of H2SO4 or FeCl3, from benzal chloride by treatment with oxygen in a radical source, and from several other miscellaneous reactions. Benzoyl chloride can be reduced to benzaldehyde, oxidized to benzoyl peroxide, chlorinated to chlorobenzoyl chloride and sulfonated to m-sulfobenzoic acid. It will undergo various reactions with organic reagents. For example, it will add across an unsaturated (alkene or alkyne) bond in the presence of a catalyst to give the phenylchloroketone: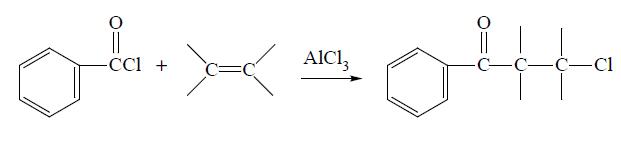
정의
ChEBI: Benzoyl chloride is an acyl chloride consisting of benzene in which a hydrogen is replaced by an acyl chloride group. It is an important chemical intermediate for the manufacture of other chemicals, dyes, perfumes, herbicides and pharmaceuticals. It has a role as a carcinogenic agent. It is an acyl chloride and a member of benzenes. It is functionally related to a benzoic acid.?일반 설명
Benzoyl chloride appears as a colorless fuming liquid with a pungent odor. Flash point 162 °F. Lachrymator, irritating to skin and eyes. Corrosive to metals and tissue. Density 10.2 lb / gal. Used in medicine and in the manufacture of other chemicals.반응 프로필
Benzoyl chloride reacts violently with protic solvents such as alcohols, with amines and amides (for example dimethylformamide [Bretherick 1979 p. 6] ) and with inorganic bases. Causes the violent decomposition of dimethyl sulfoxide [Chem. Eng. News 35(9): 87 1957]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291]. Friedel-Crafts acylation of naphthalene using Benzoyl chloride, catalyzed by AlCl3, must be conducted above the melting point of the mixture, or the reaction may be violent [Clar, E. et al., Tetrahedron, 1974, 30, 3296].위험도
Highly toxic. Strong irritant to skin, eyes, and mucous membranes, and via ingestion, inhala- tion. Upper respiratory tract irritant. Probable car- cinogen.건강위험
INHALATION: may irritate eyes, nose and throat. INGESTION: causes acute discomfort. SKIN: causes irritation and burning.화학 반응
Reactivity with Water Slow reaction with water to produce hydrochloric acid fumes. The reaction is more rapid with steam; Reactivity with Common Materials: Slow corrosion of metals but no immediate danger; Stability During Transport: Not pertinent; Neutralizing Agents for Acids and Caustics: Soda ash and water, lime; Polymerization: Does not occur; Inhibitor of Polymerization: Not pertinent.Mechanism of action
Indicative of its high reactivity (relative to alkyl chlorides), benzyl chloride reacts with water in a hydrolysis reaction to form benzyl alcohol and hydrochloric acid. In contact with mucous membranes, hydrolysis produces hydrochloric acid. Thus, benzyl chloride is a lachrymator and has been used in chemical warfare. Theoretically, for every mole of benzyl chloride reacted, one mole of hydrochloric acid is released[1].Toxicology
Benzoyl chloride is of low acute oral toxicity in rats (LD50 2529 mg/kg). It is more toxic by inhalation (LC50 230 ppm, 4 h in male rats and 314 ppm, 4 h in female rats). The compound is irritating to skin, mucous membranes, eyes, and the respiratory tract.When benzoyl chloride or solutions of benzoyl chloride in benzene were applied to the skin of mice for up to 10 months irritation and keratinization resulted, and to some extent, ulceration and necrosis of the skin occurred. A few tumors (skin, lung) were observed in those mice. There is no clear evidence that benzoyl chloride is mutagenic.
For humans, benzoyl chloride is classified as a lachrymator. It is irritating to the skin, eyes, and mucous membranes. The available data are inadequate to evaluate the carcinogenic potential of benzoyl chloride to humans.
잠재적 노출
Benzoyl chloride is used as a chemical intermediate; in organic synthesis; to produce other chemicals, dyes, perfumes, herbicides, and medicines.운송 방법
UN 1736 Benzoylchloride, Hazard class: 8; Labels: 8—Corrosive material.Purification Methods
A solution of benzoyl chloride (300mL) in *C6H6 (200mL) is washed with two 100mL portions of cold 5% NaHCO3 solution, separated, dried with CaCl2 and distilled [Oakwood & Weisgerber Org Synth III 113 1955]. Repeated fractional distillation at 4mm Hg through a glass helices-packed column (avoiding porous porcelain or silicon-carbide boiling chips, and hydrocarbon or silicon greases on the ground joints) gave benzoyl chloride that did not darken on addition of AlCl3. Further purification is achieved by adding 3 mole% each of AlCl3 and toluene, standing overnight, and distilling off the benzoyl chloride at 1-2mm [Brown & Jenzen J Am Chem Soc 80 2291 1958]. Refluxing for 2hours with an equal weight of thionyl chloride before distillation has also been used. [Beilstein 9 IV 721.] Strong IRRITANT. Use in a fume cupboard.비 호환성
May form explosive mixture with air. Contact with heat, hot surfaces, and flames causes decomposition, forming phosgene and hydrogen chloride. Water contact may be violent; forms hydrochloric acid. Reactions with amines, alcohols, alkali metals, dimethyl sulfoxide, strong oxidizers, and metal salts may be violent, causing fire and explosions. Attacks metals in the presence of moisture, forming explosive hydrogen gas. Attacks some plastics, rubber or coatings.폐기물 처리
Pour into sodium bicarbonate solution and flush to sewer.염화벤조일 준비 용품 및 원자재
원자재
준비 용품
3-oxo-3-phenyl-propanamide
N,N-Dimethylpiperidin-4-amine
APLPHA-BROMO-M-BENZOYLOXYACETOPHENONE
2-(N,N-DIETHYLAMINOCARBONYL)PHENYLBORONIC ACID
2-Methyoxy-3-methyl-2-phenyl-4H-benzo-g-pyranone
(+)-디벤조닐-D-주석산
디벤조일티아민
PHENOXYACETIC ACID
Benzoyl cyanide
2,5-DICHLOROBENZOYL CHLORIDE
7-HYDROXY-3-METHYLFLAVONE
1-BENZOYLPIPERIDINE
Proglumide
4-Chlorobenzophenone
히스타민
3,4-디아미노벤조페논
베타-D-프락토푸라노실, 알파-D-글루코피라노사이드벤조에이트
1,3-DIBENZOYLOXYBENZENE
2-퀴놀린카르보니트릴
1-NAPHTHYL PHENYL KETONE
t-부틸 퍼옥시벤조에이트
3,4-Dichlorobenzophenone
N-(4-아미노-2,5-다이에톡시페닐)벤즈아마이드
dihydroxyethyl p-octadecyl phenylsulfonyl amino propyl ammoium propylsulfonate
3-Chlorobenzoyl chloride
2-Amino-5-nitrobenzophenone
크리신
N,N'-DIBENZOYLHYDRAZINE
N-Phenylbenzohydroxamic acid
N,N-Dimethylbenzamide
페닐 벤조산염
Nitrazepam
dibenzoyl disulphide
1-(PHENYLSULFONYL)-1H-INDOLE-2-CARBALDEHYDE
2-하이드록시-4-메톡시벤조페논
4-BROMOPHENYLTHIOUREA
1-BENZOYLPIPERAZINE HYROCHLORIDE 97
2-AMINO-1-PHENYLETHANOL
Benzoylferrocene
4-클로르-o-톨루딘
염화벤조일 공급 업체
글로벌( 614)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Wuhan Biet Co., Ltd. | +8617320528784 |
min@biet.com.cn | China | 41 | 58 |
| Ningxia Jinhua Chemical Co.,Ltd | 025-52279164 |
info@nxjhchem.com | China | 79 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418684 +8618949823763 |
sales@tnjchem.com | China | 25363 | 58 |
| Taizhou Suze Chemical Materials Co.,Ltd. | +86-0523-86392777 +86-19825580222 |
nick.miao@suzechem.com | China | 23 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |
| Anhui Royal Chemical Co., Ltd. | +86-25-86655873 +8613962173137 |
marketing@royal-chem.com | China | 142 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 |
kaia@neputrading.com | China | 1011 | 58 |