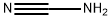시안아미드
|
|
시안아미드 속성
- 녹는점
- 45-46 °C (lit.)
- 끓는 점
- 83 °C/0.5 mmHg (lit.)
- 밀도
- 1,282 g/cm3
- 증기압
- 1Pa at 24.95℃
- 굴절률
- 1.405
- 인화점
- >230 °F
- 저장 조건
- 2-8°C
- 용해도
- 에탄올: 용해성 10%, 투명~흐릿함, 무색~미황색
- 산도 계수 (pKa)
- 1.1(at 29℃)
- 물리적 상태
- 결정질 고체
- Specific Gravity
- 1.282
- 수용성
- 775g/L
- 감도
- Hygroscopic
- Merck
- 14,2684
- BRN
- 1732569
- 노출 한도
- ACGIH: TWA 2 mg/m3
NIOSH: TWA 2 mg/m3
- 안정성
- 불안정 - 열에 민감함. 강한 산화제, 강한 환원제, 염기, 산, 철 및 그 염, 강철, 황동, 납, 습기와 호환되지 않습니다. 산과 반응하여 매우 유독한 가스를 생성합니다.
- InChIKey
- XZMCDFZZKTWFGF-UHFFFAOYSA-N
- LogP
- -0.72 at 20℃
- CAS 데이터베이스
- 420-04-2(CAS DataBase Reference)
- NIST
- Cyanamide(420-04-2)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 21-25-36/38-43 | ||
| 안전지침서 | 3-22-36/37-45-26 | ||
| 유엔번호(UN No.) | UN 2811 6.1/PG 3 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | GS5950000 | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | III | ||
| HS 번호 | 29269090 | ||
| 유해 물질 데이터 | 420-04-2(Hazardous Substances Data) | ||
| 독성 | LD50 i.p. in male mice: 200-300 mg/kg (Doull) | ||
| 기존화학 물질 | KE-09027 | ||
| 유해화학물질 필터링 | 97-1-145 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 시안아미드 및 이를 25% 이상 함유한 혼합물 |
시안아미드 C화학적 특성, 용도, 생산
개요
Cyanamide and its salts are used on various occasions, such as in chemistry, in anti-rust solutions or in drugs for treating alcoholism (Come).화학적 성질
Cyanamide is a combustible crystalline solid, but it is usually found as a 25% liquid solution.용도
In Europe, cyanamide is used as a fertilizer, weed killer, and defoliant. In North America, these applications have been practically discontinued. It is also used to produce cationic starch and calcium cyanide, dicyandiamide, and melamine. New uses include intermediates for pesticides; detergents; medicines such as antihistamines, hypertension, sedatives, and contraceptives; photography industry; additive for fuels and lubricants; paper preservative; and cement additive. Dormex is a common rest-breaking agent applied in spring to stimulate uniform opening of buds.Cyanamide has been tested as an effective and welltolerated pharmacological adjunct to treat alcohol-dependent patients. It is a potent aldehyde dehydrogenase inhibitor, and alters cholinergic function in the frontal cortex and hippocampus of rats exposed to ethanol.
생산 방법
The basic process for the manufacture of cyanamide comprises four steps. The first three steps produce calcium cyanamide: lime is made from high grade limestone; (2) calcium carbide is manufactured from lime and coal or coke; calcium cyanamide is produced by passing gaseous nitrogen through a bed of calcium carbide with 1% calcium fluorspar, which is heated to 1000–1100°C to start the reaction—the heat source is then removed and the reaction continues because of its strong exothermic character; and cyanamide is manufactured from calcium cyanamide by continuous carbonation in an aqueous medium.정의
cyanamide: 1. An inorganic saltcontaining the ion CN22-. See calciumcyanamide. 2. A colourless crystallinesolid, H2NCN, made by the actionof carbon dioxide on hotsodamide. It is a weakly acidic compound(the parent acid of cyanamidesalts) that is soluble in water andethanol. It is hydrolysed to urea inacidic solutions.화학 반응
Cyanamide reacts (1) as a base with strong acids forming salts, (2) as an acid forming metallic salts, such as calcium cyanamide CaCN2. Cyanamide is formed (1) by reaction of cyanogen chloride CN·Cl plus ammonia (ammonium chloride also formed), (2) by reaction of thiourea plus lead hydroxide (lead sulfide also formed).일반 설명
Colorless deliquescent crystals. Mp: 45°C; bp: 260°C. Density: 1.282 g cm-3. Quite soluble in water (77 g / 100 g solution at 15°C). Soluble in butanol, methyl ethyl ketone, ethyl acetate, alcohols, phenols, amines, ethers. Note: The term "Cyanamide" is also used to refer to the important compound calcium Cyanamide, which is a different chemical.반응 프로필
Cyanamide is the amide of cyanic acid. Non-flammable but combustible (flash point: 140°C). Decomposes on warming above 49°C. Emits toxic fumes of CN- and NOx when heated to decomposition or on contact with acids or acid fumes (Hazardous Chemicals Desk Reference, p. 353 (1987)). Contact with moisture, acids or bases may cause a violent reaction at temperatures above about 40°C. Dry solid may polymerize at temperatures above 122°C. Rapid or explosive polymerization may occur during the evaporation of aqueous solutions. Reacts explosively with strong oxidizing agents and strong reducing agents. Attacks various metals (International Chemical Safety Card).위험도
Strong irritant to skin and mucous membranes; avoid inhalation or ingestion.건강위험
Cyanamide is an irritant of the eyes, mucous membranes, and skin; it is an inhibitor of aldehyde dehydrogenase and can cause an “antabuse” effect with ethanol ingestion.Cyanamide is severely irritating and caustic to the eyes, skin, and respiratory tract.
농업용
Herbicide, Plant growth regulator: A U.S. EPA restricted Use Pesticide (RUP). Not currently approved for use in EU countries (re-submitted). Used primarily as a plant growth regulator. Cyanamide may be melted to give a dimer, dicyandiamide or cyanoguanidine. At higher temperatures it gives the trimer, melamine, a raw material for melamine-formaldehyde resins.상품명
DORMEX®; SKW 83010®색상 색인 번호
Cyanamide and its salts are used in various occasions such as in chemistry, in antirust solutions, or in a drug (Come?) for treating alcoholism (inhibition of alcohol deshydrogenase).Safety Profile
Poison by ingestion, inhalation, and intraperitoneal routes. Moderately toxic by skin contact. Experimental reproductive effects. Combustible when exposed to heat or flame. To fight fire, use CO2, dry chemical. Thermally unstable. Contact with moisture (water), acids, or alkalies may cause a violent reaction above 40'. Concentrated aqueous solutions may undergo explosive polymerization. Mixture with 1,2 phenylenediamine salts may cause explosive polymerization. When heated to decomposition or on contact with acid or acid fumes, it emits toxic fumes of CNand NOx. See also CYANIDE and AMIDES.잠재적 노출
Cyanamide may be melted to give a dimer, dicyandiamide or cyanoguanidine. At higher tem- peratures it gives the trimer, melamine; a raw material for melamine-form aldehyde resins.운송 방법
UN3276 Nitriles, liquid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required, Potential Inhalation Hazard (Special Provision 5).Purification Methods
Purify it by placing ca 15g in a Soxhlet thimble and extracting exhaustively (2-3hours) with two successive portions of Et2O (400mL, saturated with H2O by shaking before use) containing two drops of 1N acetic acid. Two successive portions of Et2O are used so that the NH2CN is not heated for too long. Each extract is dried over Na2SO4 (30g), then combined and evaporated under reduced pressure. The NH2CN may be stored unchanged at 0o in Et2O solution in the presence of a trace of AcOH. Extracts from several runs may be combined and evaporated together. The residue from evaporation of an Et2O solution is a colourless viscous oil which sets to a solid and can be recrystallised from a mixture of 2 parts of *C6H6 and 1 part of Et2O. Concentrating an aqueous solution of NH2CN at high temperatures causes EXPLOSIVE polymerisation. [Kurzer & Lawson Org Synth Coll Vol IV 645 1963, Pinck & Salissbury Inorg Synth III 39 1950, Soloway & Lipschitz J Org Chem 23 613 1958.] Hygroscopic.[Beilstein 3 IV 145.]비 호환성
Cyanamide may polymerize at tempera- tures above 122℃ , or on evaporation of aqueous solutions. Reacts with acids, strong oxidants, strong reducing agents such as hydrides and water, causing explosion and toxic hazard. Attacks various metals. Decomposes when heated above 49℃ C, on contact with acids, bases, 1,2-phenylene diamine salts; and moisture; producing toxic fumes includ- ing nitrogen oxides and cyanides. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reac- tions. Nitriles are generally incompatible with other oxi- dizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids .폐기물 처리
Add excess alkaline calcium hypochlorite with agitation. Flush to sewer after 24 hours. Cyanamide can also be destroyed in an incinerator equipped with afterburner and scrubber.시안아미드 준비 용품 및 원자재
원자재
준비 용품
2-AMINO-4-METHYLOXAZOLE
N-메틸-N'-니트로-N-니트로소구아니딘
1H-1,2,4-Triazole-1-carboximidamide hydrochloride
다이사이안 다이아미드
N-Cyanoimido-S,S-dimethyl-dithiocarbonate
벤설프론 메틸
중탄산아미노구아니딘
CHLOROFORMAMIDINE HYDROCHLORIDE
L-알라닌
2,4-DIAMINO-1,3,5-TRIAZINE
2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride
2,5-Diaminopyrimidine
4-AMINO-2-CHLOROPYRIMIDINE-5-CARBONITRILE
포르마미딘 하이드로클로라이드
아미트롤
Diphenyl N-cyanocarbonimidate
Oxazole-2-amine
IMINOCTADINE TRIACETATE
DL-알라닌
시안아미드 공급 업체
글로벌( 393)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Ningxia Jinhua Chemical Co.,Ltd | 025-52279164 |
info@nxjhchem.com | China | 79 | 58 |
| Anhui Royal Chemical Co., Ltd. | +86-25-86655873 +8613962173137 |
marketing@royal-chem.com | China | 142 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 994 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7845 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8619133911216 |
fanfan@kangcang.com.cn | China | 340 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |