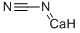석회질소
|
|
석회질소 속성
- 녹는점
- >300 °C(lit.)
- 밀도
- 2.29
- 증기압
- 0.51Pa at 20℃
- 용해도
- reacts with H2O
- 물리적 상태
- 가루
- 색상
- 회색에서 진한 회색까지
- Specific Gravity
- 2.29
- 수용성
- 불용성 H2O, 가수분해를 거쳐 아세틸렌과 암모니아가 방출됨 [HAW93] [MER06]
- 감도
- Moisture Sensitive
- Merck
- 14,1662
- BRN
- 4124391
- InChIKey
- QFSRQFUIHVTIDL-UHFFFAOYSA-N
- LogP
- -0.72 at 20℃
- CAS 데이터베이스
- 156-62-7(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-37-41-15-43-37/38 | ||
| 안전지침서 | 26-39-43-36/37/39-22-45-28 | ||
| 유엔번호(UN No.) | UN 1403 4.3/PG 3 | ||
| OEB | C | ||
| OEL | TWA: 0.5 mg/m3 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | GS6000000 | ||
| F 고인화성물질 | 9-21 | ||
| TSCA | Yes | ||
| 위험 등급 | 4.3 | ||
| 포장분류 | III | ||
| HS 번호 | 31029010 | ||
| 유해 물질 데이터 | 156-62-7(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-04501 |
석회질소 C화학적 특성, 용도, 생산
화학적 성질
Calcium cyanamide is a blackish-gray, shiny crystalline material or powder.
물리적 성질
Pure product is a colorless, hexagonal crystal or white powder. Commercial grade material may be grayish-black powder or lump (the color is due to presence of calcium carbide and other impurities); density 2.29 g/cm3; melts around 1,340°C; sublimes around 1,150 to 1,200°C on rapid heating; reacts with water.용도
Calcium Cyanamide is used as a fertilizer, herbicide, insecticide, a steel-making additive and an ore processing material. It can also be used to make thiourea, guanidine and ferrocyanides. manufacture of calcium cyanide, melamine, dicyandiamide.제조 방법
Calcium cyanamide is prepared from calcium carbide. The carbide powder is heated at about 1,000°C in an electric furnace into which nitrogen is passed for several hours. The product is cooled to ambient temperatures and any unreacted carbide is leached out cautiously with water.CaC2 + N2 → CaCN2 + C (ΔHƒ°= –69.0 kcal/mol at 25°C)
생산 방법
Calcium cyanamide was first produced commercially around 1900 as a fertilizer. The process of making calcium cyanamide involves three raw materials—coke, coal, and limestone— plus nitrogen. The limestone (calcium carbonate) is burned with coal to produce calcium oxide. The calcium oxide is then allowed to react with amorphous carbon in the furnace at 2000°C with the formation of calcium carbide (CaC2). Finely powdered calcium carbide is heated to 1000°C in an electric furnace into which pure nitrogen is passed. It is then removed and uncombined calcium carbide removed by leaching.정의
calcium cyanamide: A colourlesssolid, CaCN2, which sublimes at1300°C. It is prepared by heating calciumdicarbide at 800°C in a streamof nitrogen:CaC2(s) + N2(g) → CaCN2(s) + C(s)
The reaction has been used as amethod of fixing nitrogen in countriesin which cheap electricity isavailable to make the calcium dicarbide(the cyanamide process). Calciumcyanamide can be used as afertilizer because it reacts with waterto give ammonia and calcium carbonate:
CaCN2(s) + 3H2O(l) → CaCO3(s) +2NH3(g)
It is also used in the production ofmelamine, urea, and certain cyanidesalts.
일반 설명
A colorless to gray, odorless solid. May cause illness from ingestion. May irritate the skin. If exposed to water or high temperatures, calcium cyanamide may generate toxic and flammable fumes. Used to make pesticides and in fertilizers.공기와 물의 반응
Depending on the calcium carbide content, the cyanamide reacts with water (moisture from air or soil) to produce acetylene and hydrated calcium oxide or calcium hydroxide. Absorption of water during handling or storage of technical calcium cyanamide may cause explosion [Pieri, M. Chem. Abs. 46, 8335 1952].반응 프로필
When hydrated CALCIUM CARBIDE generates salts of calcium that are basic and are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.위험도
Fire risk with moisture or combined with calcium carbide. Skin, eye, and upper respiratory tract irritant. Questionable carcinogen.건강위험
Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.화재위험
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard.Safety Profile
Poison by ingestion, inhalation, sh contact, intravenous, and intraperitoneal routes. Moderately toxic to humans by ingestion. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. The fatal dose, by ingestion, is probably around 20 to 30 g for an adult. It does not have a cyanide effect. Calcium cyanamide is not believed to have a cumulative action. Flammable. Reaction with water forms the explosive acetylene gas. When heated to decomposition it emits toxic fumes of NOx and CN-. See also CALCIUM COMPOUNDS, AMIDES, and CYANIDE잠재적 노출
Calcium cyanamide is used in agriculture as a fertilizer, herbicide; defoliant for cotton plants; and pesticide. It is also used in the manufacture of dicyandiamide and calcium cyanide as a desulfurizer in the iron and steel industry; and in steel hardening.Carcinogenicity
Calcium cyanamide was weakly mutagenic in Salmonella typhimurium strain TA1535 and nonmutagenic in strain TA100.운송 방법
UN1403 Calcium cyanamide with .1% calcium carbide, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material비 호환성
Commercial grades of calcium cyanamide may contain calcium carbide; contact with any form of moisture solutions may cause decomposition, liberating explosive acetylene gas and ammonia. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. May polymerize in water or alkaline solutions to dicyanamide. Contact with all solvents tested also causes decomposition석회질소 준비 용품 및 원자재
원자재
준비 용품
Midinyanglin
메틸시아노카바메이트
다이사이안 다이아미드
N-Cyanoimido-S,S-dimethyl-dithiocarbonate
Black cyanide
guanidine
중탄산아미노구아니딘
카벤다짐
1,1-DIMETHYLGUANIDINE SULFATE
4-AMINO-2-METHOXYPYRIMIDINE-5-CARBONITRILE
Aminoguanidinium Carbonate
헥사지논
피리미포스-메틸
이산화티오요소
다이-n-뷰틸아민
구아니딘 나이트레이트
시안아미드
Aminoguanidinium sulphate
칼슘 질산, 테트라수화물
티오요소
석회질소 공급 업체
글로벌( 108)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12446 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21667 | 55 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2931 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8820 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Standardpharm Co. Ltd. | 86-714-3992388 |
overseasales1@yongstandards.com | United States | 14335 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39916 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29823 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34571 | 58 |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 |
info@afinechem.com | China | 15394 | 58 |