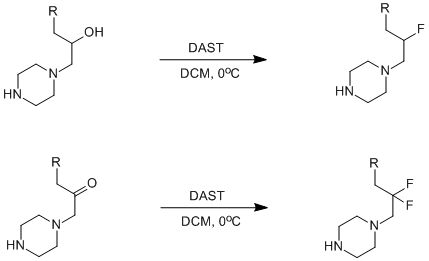
DAST二乙胺基三氟化硫,一种常用的氟化试剂。常温下是液体,在干燥情况下室温或冰箱能长期保存,DAST在90oC会分解,处理不当会有爆炸的危险。但由于操作简单和通用性强,DAST是使用得最广泛的氟代试剂之一。这个试剂能将羟基化合物转化为单氟代化合物,醛和酮转化为二氟代化合物[1]。
反应机理:
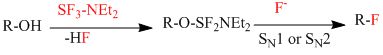
首先DAST会和羟基生成一个中间态,并生成一分子氟化氢。之后是氟负离子通过SN1或者SN2方式亲核进攻羟基碳原子,从而生成产物[2,3]。
反应特点:
脂肪族和芳香族的伯,仲,叔醇都能高产率的转化为相应的氟化合物。反应通常用二氯甲烷,一氟三氯甲烷等作溶剂,取代羟基通常在较低温度下反应(-78oC),取代羰基一般在0oC-40oC反应。
仲醇在取代的过程中会发生构型翻转,为手性合成提供了一个方法,如 (S)-2-辛醇在反应中构型完全翻转,得到 (R)-2-氟辛烷,ee%为97.6%[4]。

文献示例:

A dry, 1-L, three-necked, round-bottomed flask is fittedwith a 500-mL dropping funnel, thermometer, a magnetic stirrer,and a reflux condenser protected from theatmosphere with a drying tube. The apparatus is flushed with dry nitrogen, and 150 mL of dry dichloromethane and 21 mL (0.16mole) of diethylaminosulfurtrifluoride[5] are added to the flask. The contents of the flask are cooled to 10°, and a solution of 23 g (0.150 mole) of 4-nitrobenzyl alcohol in 450 mL of dichloromethane is added dropwise at a fast rate (45 minutes). The reaction mixture is allowed to come toroom temperature and poured into a beakercontaining 300 gof ice, decomposing any unreacted diethylaminosulfurtrifluoride. The organic layer isseparated, and the water layer is extracted twice with 45-mL portions of dichloromethane. The organic layer andextracts are combined, washed with 150 mL of water, and dried over anhydrous magnesium sulfate. Evaporation to dryness under reduced pressure gives 20.9–22.1 g(90–95%) of crude product. Recrystallization from 500 mL of pentane yields 15.5 g. (67%) of 4-nitrobenzyl fluoride as colorless needle-shapedcrystals, m.p. 36–37°
作者示例:
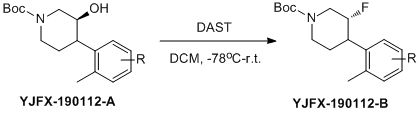
YJFX-190112-A (10.0 g, 1.0 eq.) was dissolved in CH2Cl2(100 mL), DAST (5.2 g, 1.2 eq.) was dropped slowly into the solutionunder -78 °C. The mixture was stirred for 30 min at -78 °C. The reaction waswarmmed to 30-40 °C and stirred for 1-2h.
Themixture was dropped into aq. NaHCO3 (10%, 200mL), and stirred for 20min. Extracted with CH2Cl2 (150mL*2), the organic layerwas dried with anhydrous Na2SO4 and concentrated, and the residuewas purified by column chromatography togive product as oil (8.5 g, yield: 84%).
反应经验:
带有手性的仲醇,发生了构型翻转。
该反应滴加DAST在零下78摄氏度进行,而中间态往产品的转化却需要升温进行,因此升温时必要的(不同底物需要自己摸索了)。上述反应的中间态极性和产品几乎几乎无差别(TLC检测无法分别),导致最初反应做的差的原因,延长时间后,得到改善。
反应淬灭过程建议滴加,上述反应最开始是倒入,反应会瞬间变杂,控制温度也不行,改为滴加后,得到改善。
参考文献:
Bombrun, A. et. al. J. Med. Chem. 2003, 46,4365.
Rozen, S. ; Faust, Y. ; Ben-Yakov, H. Tetrahedron Lett. 1979,20, 1823.
Leroy, J.; Hebert, E.; Wakselman, C. J. Org. Chem. 1979, 44,3406.
Middleton, W, J. J. Org. Chem. 1975, 40, 574.
Hundlicky, M. Org. React. 1988, 34, 513.
