Oxygen
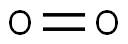
|
9 prices
Selected condition:
Brand
- American Custom Chemicals Corporation
- Rare Earth Products
- Sigma-Aldrich
- Usbiological
Package
- 5mg
- 100UL
- 25gm
- 1ea
- 100gm
- 56L
- 25μL
- 100μL
- ManufacturerAmerican Custom Chemicals Corporation
- Product numberGAS0000005
- Product descriptionOXYGEN 95.00%
- Packaging56L
- Price$6603.14
- Updated2021-12-16
- Buy
- ManufacturerRare Earth Products
- Product numberE0932K
- Product descriptionOxygen
- Packaging25gm
- Price$39
- Updated2021-12-16
- Buy
- ManufacturerRare Earth Products
- Product numberE0932K
- Product descriptionOxygen
- Packaging100gm
- Price$145
- Updated2021-12-16
- Buy
- ManufacturerSigma-Aldrich
- Product number300500-U
- Product descriptionOxygen (99.6%) 99.6%, cylinder of 14?L, analytical standard
- Packaging1ea
- Price$219
- Updated2022-05-15
- Buy
- ManufacturerSigma-Aldrich
- Product numberHPA043930
- Product descriptionAnti-LOX antibody produced in rabbit Prestige Antibodies? Powered by Atlas Antibodies, affinity isolated antibody
- Packaging25μL
- Price$282
- Updated2023-06-20
- Buy
- ManufacturerSigma-Aldrich
- Product numberSAB5700990
- Product descriptionAnti-LOX antibody produced in rabbit
- Packaging100UL
- Price$389
- Updated2023-06-20
- Buy
- ManufacturerSigma-Aldrich
- Product numberSAB5700990
- Product descriptionAnti-LOX antibody produced in rabbit
- Packaging100μL
- Price$418
- Updated2025-07-31
- Buy
- ManufacturerSigma-Aldrich
- Product numberHPA043930
- Product descriptionAnti-LOX antibody produced in rabbit Prestige Antibodies? Powered by Atlas Antibodies, affinity isolated antibody
- Packaging100μL
- Price$555
- Updated2025-07-31
- Buy
- ManufacturerUsbiological
- Product number282071
- Product descriptionO-2-
- Packaging5mg
- Price$403
- Updated2021-12-16
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| American Custom Chemicals Corporation | GAS0000005 | OXYGEN 95.00% | 56L | $6603.14 | 2021-12-16 | Buy |
| Rare Earth Products | E0932K | Oxygen | 25gm | $39 | 2021-12-16 | Buy |
| Rare Earth Products | E0932K | Oxygen | 100gm | $145 | 2021-12-16 | Buy |
| Sigma-Aldrich | 300500-U | Oxygen (99.6%) 99.6%, cylinder of 14?L, analytical standard | 1ea | $219 | 2022-05-15 | Buy |
| Sigma-Aldrich | HPA043930 | Anti-LOX antibody produced in rabbit Prestige Antibodies? Powered by Atlas Antibodies, affinity isolated antibody | 25μL | $282 | 2023-06-20 | Buy |
| Sigma-Aldrich | SAB5700990 | Anti-LOX antibody produced in rabbit | 100UL | $389 | 2023-06-20 | Buy |
| Sigma-Aldrich | SAB5700990 | Anti-LOX antibody produced in rabbit | 100μL | $418 | 2025-07-31 | Buy |
| Sigma-Aldrich | HPA043930 | Anti-LOX antibody produced in rabbit Prestige Antibodies? Powered by Atlas Antibodies, affinity isolated antibody | 100μL | $555 | 2025-07-31 | Buy |
| Usbiological | 282071 | O-2- | 5mg | $403 | 2021-12-16 | Buy |
Properties
Melting point :−218 °C(lit.)
Boiling point :−183 °C(lit.)
Density :1.429(0℃)
vapor density :1.11 (vs air)
vapor pressure :>760 mmHg at 20 °C
storage temp. :-20°C
solubility :At 20 °C and at a pressure of 101 kPa, 1 volume dissolves in about 32 volumes of water.
form :colorless gas
color :Colorless gas, liquid, or hexagonal crystals
Odor :Odorless gas
biological source :rabbit
Water Solubility :one vol gas dissolves in 32 volumes H2O (20°C), in 7 volumes alcohol (20°C); soluble other organic liq, usually higher solubility than in H2O [MER06]
Merck :13,7033
Dielectric constant :1.5(-193℃)
Stability :Stable. Vigorously supports combustion. Incompatible with phosphorus, organic materials, many powdered metals.
Cosmetics Ingredients Functions :SKIN CONDITIONING
InChI :1S/O2/c1-2/i1+0,2+0
InChIKey :MYMOFIZGZYHOMD-ZCWHFVSRSA-N
SMILES :[16O]=[16O]
Surface tension :15.85 mN/m at 80.0K
CAS DataBase Reference :7782-44-7(CAS DataBase Reference)
NIST Chemistry Reference :Oxygen(7782-44-7)
EPA Substance Registry System :Oxygen (7782-44-7)
Boiling point :−183 °C(lit.)
Density :1.429(0℃)
vapor density :1.11 (vs air)
vapor pressure :>760 mmHg at 20 °C
storage temp. :-20°C
solubility :At 20 °C and at a pressure of 101 kPa, 1 volume dissolves in about 32 volumes of water.
form :colorless gas
color :Colorless gas, liquid, or hexagonal crystals
Odor :Odorless gas
biological source :rabbit
Water Solubility :one vol gas dissolves in 32 volumes H2O (20°C), in 7 volumes alcohol (20°C); soluble other organic liq, usually higher solubility than in H2O [MER06]
Merck :13,7033
Dielectric constant :1.5(-193℃)
Stability :Stable. Vigorously supports combustion. Incompatible with phosphorus, organic materials, many powdered metals.
Cosmetics Ingredients Functions :SKIN CONDITIONING
InChI :1S/O2/c1-2/i1+0,2+0
InChIKey :MYMOFIZGZYHOMD-ZCWHFVSRSA-N
SMILES :[16O]=[16O]
Surface tension :15.85 mN/m at 80.0K
CAS DataBase Reference :7782-44-7(CAS DataBase Reference)
NIST Chemistry Reference :Oxygen(7782-44-7)
EPA Substance Registry System :Oxygen (7782-44-7)
Safety Information
| Symbol(GHS): |
 
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Danger | |||||||||||||||||||||
| Hazard statements: |
|
|||||||||||||||||||||
| Precautionary statements: |
|
Description
Oxygen is a very prevalent and important element and is necessary for sustaining life on this planet. This element is the third most abundant in mass behind helium and hydrogen in the universe. The diatomic form (O2) is the most common pure form. With a boiling point at -183 °C, O2 exists as a colorless and odorless gas at standard temperature and pressure. In the process of cellular respiration, the highly reactive O2 is used as the oxidant in breaking down food molecules to produce energy. In turn, photosynthetic organisms generate O2 by using energy from the sun and water. Other allotropes of pure oxygen exist, including the trioxygen (O3) form known as ozone, as well as other, less common allotropes of oxygen such as O4 and O8. These oxygen allotropes are formed under high pressure and low temperatures and are solid.More related product prices
Levofloxacin hydrochloride Ofloxacin Cefdinir 38821-53-3 Lacidipine Cefuroxime sodium Doxorubicin hydrochlorideRelated product price
- Levofloxacin hydrochloride
$5-1504.5 - Ofloxacin
$5-1100 - Cefotaxime sodium
$8-1790
Suppliers and manufacturers
Henan Tianfu Chemical Co.,Ltd.
Hubei xin bonus chemical co. LTD
Hebei Fengjia New Material Co., Ltd
PT CHEM GROUP LIMITED
XIAMEN AMITY INDUSTRY AND TRADE CO., LTD.
Mainchem Co., Ltd.
Chemwill Asia Co.,Ltd.
Wuhan eastop Technology Co., ltd.6
China Isotope Development Co.,Ltd






