1,4-CUBANEDICARBOXYLIC ACID manufacturers
|
| | 1,4-CUBANEDICARBOXYLIC ACID Basic information |
| Product Name: | 1,4-CUBANEDICARBOXYLIC ACID | | Synonyms: | 1,4-CUBANEDICARBOXYLIC ACID;Cubane-1,4-dicarboxylic acid;Pentacyclo[4.2.0.02,5.03,8.04,7]octane-1,4-dicarboxylic acid;cubane-1,8-dicarboxylic acid;1,4-Cubanedicarboxylic acid >=97%;(1S,2R,3R,8S)-Cubane-1,4-dicarboxylic acid;1,4-Dicarboxycubane;(1r,2R,3r,8S)-cubane-1,4-dicarboxylic acid | | CAS: | 32846-66-5 | | MF: | C10H8O4 | | MW: | 192.17 | | EINECS: | | | Product Categories: | | | Mol File: | 32846-66-5.mol | 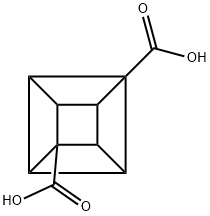 |
| | 1,4-CUBANEDICARBOXYLIC ACID Chemical Properties |
| Melting point | 224°C | | Boiling point | 457.4±45.0 °C(Predicted) | | density | 2.401±0.06 g/cm3(Predicted) | | Fp | 244.6℃ | | storage temp. | Sealed in dry,Room Temperature | | form | powder | | pka | 3.97±0.40(Predicted) | | Appearance | White to off-white Solid | | InChI | InChI=1S/C10H8O4/c11-7(12)9-1-2-4(9)6-5(9)3(1)10(2,6)8(13)14/h1-6H,(H,11,12)(H,13,14) | | InChIKey | JFKXMJUMOJJTCQ-AWFRWABZSA-N | | SMILES | C12(C(O)=O)C3C4C5(C(O)=O)C3C1C5C42 | | CAS DataBase Reference | 32846-66-5 |
| Hazard Codes | T | | Risk Statements | 25 | | Safety Statements | 45 | | RIDADR | UN 2810 6.1 / PGIII | | HS Code | 29172090 |
| | 1,4-CUBANEDICARBOXYLIC ACID Usage And Synthesis |
| Uses | 1,4-Cubanedicarboxylic acid can undergo esterification with alkylsulfuric acids. The acid and its ester derivatives are also capable of undergoing fluorination with elemental fluorine. This product was previously listed as BML00135. | | Synthesis | The general procedure for the synthesis of 1,4-cubanedicarboxylic acid from dimethyl cubane-1,4-dicarboxylate was as follows: a methanolic solution of 2.5 M NaOH (7.60 mL, 19.1 mmol, 1.05 eq.) was added slowly and dropwise to a tetrahydrofuran (THF,) solution of dimethyl cubane-1,4-dicarboxylate (4.00 g, 18.2 mmol) at room temperature. 125 mL) solution. The reaction mixture was stirred at room temperature for 14 hours. After completion of the reaction, the solvent was removed by evaporation under vacuum without heat. The residue was diluted with deionized water (45 mL) and extracted with chloroform (3 x 15 mL). The aqueous phase was acidified with 32% aqueous hydrochloric acid to pH 3. The acidified aqueous phase was re-extracted with chloroform (1 × 60 mL, 2 × 35 mL). All organic phases were combined and dried with anhydrous magnesium sulfate (MgSO4). The dried organic phase was evaporated from the solvent under reduced pressure to give 1,4-cubanedicarboxylic acid (3.46 g, 92% yield) as a colorless crystalline solid. The melting point of the product was determined to be 183-184.5 °C (literature value: 182-183 °C).1H NMR (200.1 MHz, CDCl3) data were as follows: δ 4.30-4.24 (6H, m, CH), 3.72 (3H, s, CH3), which is in agreement with the spectral data reported in the literature. | | References | [1] Synthesis, 1995, # 5, p. 501 - 502
[2] Thermochimica Acta, 2010, vol. 499, # 1-2, p. 15 - 20
[3] Journal of Organic Chemistry, 1989, vol. 54, # 24, p. 5783 - 5788
[4] Organic Letters, 2014, vol. 16, # 16, p. 4094 - 4097
[5] Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 12, p. 4059 - 4063 |
| | 1,4-CUBANEDICARBOXYLIC ACID Preparation Products And Raw materials |
|