- RIFAMYCIN
-

- $10.00 / 1Kg/Bag
-
2021-11-19
- CAS:6998-60-3
- Min. Order: 1Kg/Bag
- Purity: 99%
- Supply Ability: 20 Tons
- RIFAMYCIN SV
-

- $1.00 / 1g
-
2021-07-20
- CAS:6998-60-3
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 50tons
- rifamycin SV
-
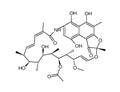
- $1.00 / 1KG
-
2020-02-05
- CAS:6998-60-3
- Min. Order: 1KG
- Purity: Min98% HPLC
- Supply Ability: g/kg/ton
|
| | RIFAMYCIN SV Basic information |
| Product Name: | RIFAMYCIN SV | | Synonyms: | RIFAMYCIN SV;17,19,21-hexahydroxy-23-methoxy-2,4,12,16,18,20,22-heptamethyl-21-acetate;rifamicinesv;rifocin;rifocyn;Rifamycin S-Na;Rifaximin EP Impurity C;19,21,25(29),26-octaen-13-yl acetate | | CAS: | 6998-60-3 | | MF: | C37H47NO12 | | MW: | 697.77 | | EINECS: | 230-273-3 | | Product Categories: | | | Mol File: | 6998-60-3.mol | 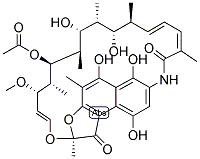 |
| | RIFAMYCIN SV Chemical Properties |
| Melting point | 300° (dec 140°) | | alpha | D20 -4° (methanol) | | Boiling point | 701.9°C (rough estimate) | | density | 1.2275 (rough estimate) | | refractive index | 1.5350 (estimate) | | pka | 5.17±0.70(Predicted) |
| Toxicity | LD50 in mice (mg/kg): 550 i.v.; 625 i.p.; 2120 orally (Bergamini, Fowst) |
| | RIFAMYCIN SV Usage And Synthesis |
| Uses | Rifogal (Rifaximin EP Impurity C NA) is a antibacterial drug which functions by inhibiting bacterial RNA polymerase (RNAP), is an important part of the antibacteral armamentarium. Also, it is one of the few drugs in a multidrug regimens for treating lung disease (LD) due to Mycobacterium avium complex (MAC). | | Definition | ChEBI: A member of the class of rifamycins that exhibits antibiotic and antitubercular properties. | | Pharmaceutical Applications | The simplest rifamycin in clinical use, obtained by elimination of a glycolic moiety from rifamycin B. Formulated as sodium salt for parenteral administration. Also available for topical use. Its activity, general properties and pharmacokinetics are very similar to those of rifamide. It is orally absorbed and excreted mainly in the bile. Intramuscular doses of 250 mg produce mean plasma levels of about 2 mg/L. The plasma half-life is around 2 h.
In addition to uses similar to those of rifamide, it is administered topically in surgery and has been proposed for the treatment of synovitis by intra-articular injections. A topical preparation is used for application to wounds and bedsores. Cases of anaphylactic reactions have been reported after local administration of the drug. | | Purification Methods | Rifamycin SV gives yellow-orange crystals from Et2O/pet ether or aqueous EtOH, is very soluble in MeOH, EtOH, Me2CO and EtOAc, and is less soluble in Et2O and HCO3-, but slightly soluble in H2O and pet ether. Its UV has max at 223, 314 and 445nm (� 1cm 586, 322 and 204) in phosphatebuffer pH 7. [NMR: Bergamini & Fowst Arzneim.-Forsch 15 951 1965.] |
| | RIFAMYCIN SV Preparation Products And Raw materials |
|