- LONAFARNIB
-

- $2.00 / 1kg
-
2019-07-06
- CAS:193275-84-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100kg
Related articles - How is Lonafarnib synthesised?
- Lonafarnib was synthesised starting from the bromination of chloroanthranilic acid and using the lonafarnib precursor tricycle....
- Dec 27,2023
|
| | LONAFARNIB Basic information |
| Product Name: | LONAFARNIB | | Synonyms: | LONAFARNIB;4-(2-(4-((R)-3,10-dibroMo-8-chloro-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-yl)cyclohexyl)-2-oxoethyl)piperidine-1-carboxaMide;Lonafarnib (SCH66336);1-Piperidinecarboxamide, 4-(2-(4-((11R)-3,10-dibromo-8-chloro-6,11-dihydro-5H-benzo(5,6)cyclohepta(1,2-B)pyridin-11-yl)-1-piperidinyl)-2-oxoethyl)-;4-(2-(4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H-benzo-(5,6)-cyclohepta(1,2-B)-pyridin-11(R)-yl)-1-piperidinyl)-2-oxo-ethyl)-1-piperidinecarboxamide;Lonafarnib [usan];Sarasar;Sch 66336 | | CAS: | 193275-84-2 | | MF: | C27H31Br2ClN4O2 | | MW: | 638.82 | | EINECS: | | | Product Categories: | Inhibitor;Aromatics;Heterocycles;Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;APIs | | Mol File: | 193275-84-2.mol | 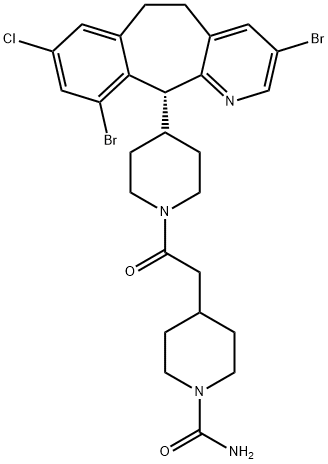 |
| | LONAFARNIB Chemical Properties |
| Melting point | 214.5-215.9° (monohydrate); mp 222-223° | | alpha | D25 = +49.1° (c = 0.21 in methanol) | | Boiling point | 710.4±70.0 °C(Predicted) | | density | 1.536 | | storage temp. | -20°C | | solubility | Chloroform (Slightly), Methanol (Slightly) | | pka | 15.76±0.40(Predicted) | | form | powder | | color | white to beige |
| | LONAFARNIB Usage And Synthesis |
| Uses | Lonafarnib is an orally bioavailable tricyclic inhibitor of farnesyl protein transferase. It inhibits Rheb farnesylation and mTOR signaling and enhances taxane and tamoxifen antitumor activity. Studies show that it induces CCAAT/enhancer-binding protein homologous protein-dependent expression of death receptor 5, leading to induction of apoptosis in human cancer cells | | Uses | Chemotherapeutic (farnesyl transfer ase inhibitor). | | Definition | ChEBI: A 4-{2-[4-(3,10-dibromo-8-chloro-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-yl)piperidin-1-yl]-2-oxoethyl}piperidine-1-carboxamide that has R configuration. It is used as oral farnesyltransferase
nhibitor. | | General Description | Lonafarnib (SCH66336) is a farnesyl transferase inhibitor (FTI). K- and N-Ras are substrates of farnesyl transferase. | | Biological Activity | lonafarnib (sch66336, sarasar) is an potent, selective, orally, bioavailable tricyclic nonpeptidyl nonsulfhydry inhibitor of farnesyltransferase (ftase).[1] it is a small molecular with the formula of c27h31br2cln4o2 and molecular weight of 638.82. farnesylated ras proteins was found to regulate signal transduction pathways which drive cell proliferation, growth and survival and be required for its membrane localization.[1, 2] lonafarnib inhibits the post-translational farnesylcation of ras proteins, therefore blocking translocation of ras to the plasma membrane.[3][1] eric w, malcolm j. m, kim n. c, d. scott e, et al. a multinomial phase ii study of lonafarnib (sch 66336) in patients with refractory urothelial cancer. urologic oncology: seminars and original investigations. 2005, 23. 143-149.[2] gongjie l, stacey a. t, cindy h. m, yunsheng h, w. robert b, et al. continuous and intermittent dosing of lonafarnib potentiates the therapeutic efficacy of docetaxel on preclinical human prostate cancer models. int. j. cancer. 2009, 125. 2711–2720.[3] vasiliki a. n, alexander j. s, keith t. f, hensin t, et al. melanoma: new insights and new therapies. j invest dermatol. 2012, 132. 854–863. | | Biochem/physiol Actions | Lonafarnib prevents the post-translational lipid modification of H-Ras and other farnesylated proteins. Lonafarnib treatment results in microtubule bundling, increased microtubule acetylation and stabilization and suppression of microtubule dynamics. | | Mechanism of action | Lonafarnib is a protein farnesyltransferase inhibitor (FTI) that reversibly binds to the farnesyltransferase CAAX binding site9, thereby inhibiting progerin farnesylation and subsequent intercalation into the nuclear membrane. | | Side effects | - vomiting
- diarrhea
- nausea
- stomach pain
- constipation
- gas
- decreased appetite
- decreased weight
| | target | FT | | storage | Store at -20°C |
| | LONAFARNIB Preparation Products And Raw materials |
|